Mycoplasma pneumoniae Community Acquired Respiratory Distress Syndrome toxin expression reveals growth phase and infection-dependent regulation
- PMID: 20199607
- PMCID: PMC2883071
- DOI: 10.1111/j.1365-2958.2010.07092.x
Mycoplasma pneumoniae Community Acquired Respiratory Distress Syndrome toxin expression reveals growth phase and infection-dependent regulation
Abstract
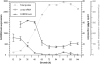
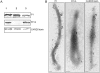
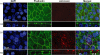
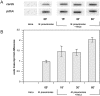
Mycoplasma pneumoniae causes acute and chronic respiratory infections, including tracheobronchitis and community acquired pneumonia, and is linked to asthma and an array of extra-pulmonary disorders. Recently, we identified an ADP-ribosylating and vacuolating toxin of M. pneumoniae, designated Community Acquired Respiratory Distress Syndrome (CARDS) toxin. In this study we analysed CARDS toxin gene (annotated mpn372) transcription and identified its promoter. We also compared CARDS toxin mRNA and protein profiles in M. pneumoniae during distinct in vitro growth phases. CARDS toxin mRNA expression was maximal, but at low levels, during early exponential growth and declined sharply during mid-to-late log growth phases, which was in direct contrast to other mycoplasma genes examined. Between 7% and 10% of CARDS toxin was localized to the mycoplasma membrane at mid-exponential growth, which was reinforced by immunogold electron microscopy. No CARDS toxin was released into the medium. Upon M. pneumoniae infection of mammalian cells, increased expression of CARDS toxin mRNA was observed when compared with SP-4 broth-grown cultures. Further, confocal immunofluorescence microscopy revealed that M. pneumoniae readily expressed CARDS toxin during infection of differentiated normal human bronchial epithelial cells. Analysis of M. pneumoniae-infected mouse lung tissue revealed high expression of CARDS toxin per mycoplasma cell when compared with M. pneumoniae cells grown in SP-4 medium alone. Taken together, these studies indicate that CARDS toxin expression is carefully controlled by environmental cues that influence its transcription and translation. Further, the acceleration of CARDS toxin synthesis and accumulation in vivo is consistent with its role as a bona fide virulence determinant.
Figures
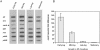



References
-
- Barile MF, Chandler DK, Yoshida H, Grabowski MW, Harasawa R, Ahmed OA. Hamster challenge potency assay for evaluation of Mycoplasma pneumoniae vaccines. Isr J Med Sci. 1981;17:682–686. - PubMed
-
- Baseman JB, Lange M, Criscimagna NL, Giron JA, Thomas CA. Interplay between mycoplasmas and host target cells. Microb Pathog. 1995;19:105–116. - PubMed
-
- Chang LJ, Chen WH, Minion FC, Shiuan D. Mycoplasmas regulate the expression of heat-shock protein genes through CIRCE-HrcA interactions. Biochem Biophys Res Commun. 2008;367:213–218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

