A coupled level set framework for bladder wall segmentation with application to MR cystography
- PMID: 20199924
- PMCID: PMC2894540
- DOI: 10.1109/TMI.2009.2039756
A coupled level set framework for bladder wall segmentation with application to MR cystography
Abstract
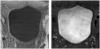
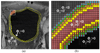
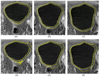
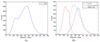
In this paper, we propose a coupled level set (LS) framework for segmentation of bladder wall using T(1)-weighted magnetic resonance (MR) images with clinical applications to virtual cystoscopy (i.e., MR cystography). The framework uses two collaborative LS functions and a regional adaptive clustering algorithm to delineate the bladder wall for the wall thickness measurement on a voxel-by-voxel basis. It is significantly different from most of the pre-existing bladder segmentation work in four aspects. First of all, while most previous work only segments the inner border of the wall or at most manually segments the outer border, our framework extracts both the inner and outer borders automatically except that the initial seed point is given by manual selection. Secondly, it is adaptive to T(1)-weighted images with decreased intensities in urine, as opposed to enhanced intensities in T(2)-weighted scenario and computed tomography. Thirdly, by considering the image global intensity distribution and local intensity contrast, the defined image energy function in the framework is more immune to inhomogeneity effect, motion artifacts and image noise. Finally, the bladder wall thickness is measured by the length of integral path between the two borders which mimic the electric field line between two iso-potential surfaces. The framework was tested on six datasets with comparison to the well-known Chan-Vese (C-V) LS model. Five experts blindly scored the segmented inner and outer borders of the presented framework and the C-V model. The scores demonstrated statistically the improvement in detecting the inner and outer borders.
Figures













Similar articles
-
Volume-based features for detection of bladder wall abnormal regions via MR cystography.IEEE Trans Biomed Eng. 2011 Sep;58(9):2506-12. doi: 10.1109/TBME.2011.2158541. Epub 2011 Jun 2. IEEE Trans Biomed Eng. 2011. PMID: 21642039 Free PMC article.
-
Quantitative Analysis of Bladder Wall Thickness for Magnetic Resonance Cystoscopy.IEEE Trans Biomed Eng. 2015 Oct;62(10):2402-9. doi: 10.1109/TBME.2015.2429612. Epub 2015 May 5. IEEE Trans Biomed Eng. 2015. PMID: 25955985
-
A unified EM approach to bladder wall segmentation with coupled level-set constraints.Med Image Anal. 2013 Dec;17(8):1192-205. doi: 10.1016/j.media.2013.08.002. Epub 2013 Aug 16. Med Image Anal. 2013. PMID: 24001932 Free PMC article.
-
Bladder tumors: virtual MR cystoscopy.Abdom Imaging. 2006 Jul-Aug;31(4):483-9. doi: 10.1007/s00261-005-0132-z. Abdom Imaging. 2006. PMID: 16568363 Review.
-
Self-adaptive weighted level set evolution based on local intensity difference for parotid ducts segmentation.Comput Biol Med. 2019 Nov;114:103432. doi: 10.1016/j.compbiomed.2019.103432. Epub 2019 Sep 4. Comput Biol Med. 2019. PMID: 31521897 Review.
Cited by
-
Motion correction for MR cystography by an image processing approach.IEEE Trans Biomed Eng. 2013 Sep;60(9):2401-10. doi: 10.1109/TBME.2013.2257769. Epub 2013 Apr 12. IEEE Trans Biomed Eng. 2013. PMID: 23591468 Free PMC article.
-
Deep-learning convolutional neural network: Inner and outer bladder wall segmentation in CT urography.Med Phys. 2019 Feb;46(2):634-648. doi: 10.1002/mp.13326. Epub 2019 Jan 4. Med Phys. 2019. PMID: 30520055 Free PMC article.
-
Study Progress of Noninvasive Imaging and Radiomics for Decoding the Phenotypes and Recurrence Risk of Bladder Cancer.Front Oncol. 2021 Jul 15;11:704039. doi: 10.3389/fonc.2021.704039. eCollection 2021. Front Oncol. 2021. PMID: 34336691 Free PMC article. Review.
-
The effect of different adipose tissue measurements on clinical prognosis in bladder cancer patients undergoing radical cystectomy: preliminary results.Abdom Radiol (NY). 2025 Sep;50(9):4224-4234. doi: 10.1007/s00261-025-04838-7. Epub 2025 Feb 13. Abdom Radiol (NY). 2025. PMID: 39939543
-
Haustral fold segmentation with curvature-guided level set evolution.IEEE Trans Biomed Eng. 2013 Feb;60(2):321-31. doi: 10.1109/TBME.2012.2226242. Epub 2012 Oct 26. IEEE Trans Biomed Eng. 2013. PMID: 23193228 Free PMC article.
References
-
- Jemal A, Thomas A, Murray T, Thun M. Cancer statistics. A Cancer Journal for Clinicians. 2002;52:23–47. - PubMed
-
- Shaw ST, Poon SY, Wong ET. Routine urinalysis: is the dipstick enough? The Journal of the American Medical Association. 1985;253:1596–1600. - PubMed
-
- Lamm DL, Torti FM. Bladder cancer. A Cancer Journal for Clinicians. 1996;46:93–112. - PubMed
-
- Vining DJ, Zagoria RJ, Liu K, Stelts D. CT cystoscopy: an innovation in bladder imaging. American Journal of Roentgenology. 1996;166:409–410. - PubMed
-
- Hussain S, Loeffler JA, Babayan RK, Fenlon HM. Thin-section helical computer tomography of the bladder: initial clinical experience with virtual reality imaging. Urology. 1997;50(5):685–689. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

