Origin of active states in local neocortical networks during slow sleep oscillation
- PMID: 20200108
- PMCID: PMC2951844
- DOI: 10.1093/cercor/bhq009
Origin of active states in local neocortical networks during slow sleep oscillation
Abstract
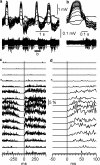
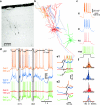
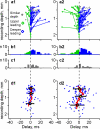
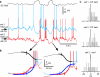
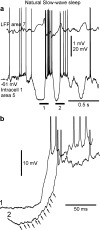
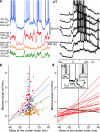
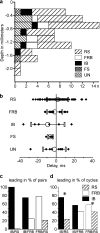
Slow-wave sleep is characterized by spontaneous alternations of activity and silence in corticothalamic networks, but the causes of transition from silence to activity remain unknown. We investigated local mechanisms underlying initiation of activity, using simultaneous multisite field potential, multiunit recordings, and intracellular recordings from 2 to 4 nearby neurons in naturally sleeping or anesthetized cats. We demonstrate that activity may start in any neuron or recording location, with tens of milliseconds delay in other cells and sites. Typically, however, activity originated at deep locations, then involved some superficial cells, but appeared later in the middle of the cortex. Neuronal firing was also found to begin, after the onset of active states, at depths that correspond to cortical layer V. These results support the hypothesis that switch from silence to activity is mediated by spontaneous synaptic events, whereby any neuron may become active first. Due to probabilistic nature of activity onset, the large pyramidal cells from deep cortical layers, which are equipped with the most numerous synaptic inputs and large projection fields, are best suited for switching the whole network into active state.
Figures




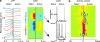
References
-
- Blake H, Gerard RW. Brain potentials during sleep. Am J Physiol. 1937;119:692–703.
-
- Chauvette S, Volgushev M, Mukovski M, Timofeev I. Local origin and long-range synchrony of active state in neocortex during slow oscillation. In: Timofeev I, editor. Mechanisms of spontaneous active states in the neocortex. Kerala (India): Research Signpost; 2007. pp. 73–92.
-
- Compte A, Sanchez-Vives MV, McCormick DA, Wang X-J. Cellular and network mechanisms of slow oscillatory activity (<1 Hz) and wave propagations in a cortical network model. J Neurophysiol. 2003;89:2707–2725. - PubMed
-
- Connors BW, Gutnick MJ. Intrinsic firing patterns of divers neocortical neurons. Trends Neurosci. 1990;13:99–104. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

