Effects of bladder outlet obstruction on properties of Ca2+-activated K+ channels in rat bladder
- PMID: 20200132
- PMCID: PMC2867513
- DOI: 10.1152/ajpregu.00523.2009
Effects of bladder outlet obstruction on properties of Ca2+-activated K+ channels in rat bladder
Abstract
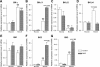
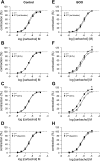
In this study, we investigated the effects of bladder outlet obstruction (BOO) on the expression and function of large conductance (BK) and small conductance (SK) Ca(2+)-activated K(+) channels in detrusor smooth muscle. The bladder from adult female Sprague-Dawley rats with 6-wk BOO were used. The mRNA expression of the BK channel alpha-subunit, beta1-, beta2-, and beta4-subunits and SK1, SK2, and SK3 channels were investigated using real-time RT-PCR. All subunits except for the BK-beta2, SK2, and SK3 channels were predominantly expressed in the detrusor smooth muscle rather than in the mucosa. The mRNA expression of the BK channel alpha-subunit was not significantly changed in obstructed bladders. However, the expression of the BK channel beta1-subunit and the SK3 channel was remarkably increased in obstructed bladders. On the other hand, the expression of the BK channel beta4-subunit was decreased as the severity of BOO-induced bladder overactivity progressed. In detrusor smooth muscle strips from obstructed bladders, blockade of BK channels by iberiotoxin (IbTx) or charybdotoxin (CTx) and blockade of SK channels by apamin increased the amplitude of spontaneous contractions. These blockers also increased the contractility and affinity of these strips for carbachol during cumulative applications. The facilitatory effects elicited by these K(+) channel blockers were larger in the strips from obstructed bladders compared with control bladders. These results suggest that long-term exposure to BOO for 6 wk enhances the function of both BK and SK types of Ca(2+)-activated K(+) channels in the detrusor smooth muscle to induce an inhibition of bladder contractility, which might be a compensatory mechanism to reduce BOO-induced bladder overactivity.
Figures



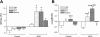
Similar articles
-
Partial bladder outlet obstruction is associated with decreased expression and function of the small-conductance Ca2+-activated K+ channel in guinea pig detrusor smooth muscle.Int Urol Nephrol. 2017 Jan;49(1):17-26. doi: 10.1007/s11255-016-1455-0. Epub 2016 Nov 14. Int Urol Nephrol. 2017. PMID: 27844406
-
NS309 decreases rat detrusor smooth muscle membrane potential and phasic contractions by activating SK3 channels.Br J Pharmacol. 2013 Apr;168(7):1611-25. doi: 10.1111/bph.12049. Br J Pharmacol. 2013. PMID: 23145946 Free PMC article.
-
Detrusor overactivity is associated with downregulation of large-conductance calcium- and voltage-activated potassium channel protein.Am J Physiol Renal Physiol. 2010 Jun;298(6):F1416-23. doi: 10.1152/ajprenal.00595.2009. Epub 2010 Apr 14. Am J Physiol Renal Physiol. 2010. PMID: 20392804 Free PMC article.
-
Central role of the BK channel in urinary bladder smooth muscle physiology and pathophysiology.Am J Physiol Regul Integr Comp Physiol. 2014 Sep 15;307(6):R571-84. doi: 10.1152/ajpregu.00142.2014. Epub 2014 Jul 2. Am J Physiol Regul Integr Comp Physiol. 2014. PMID: 24990859 Free PMC article. Review.
-
A BK (Slo1) channel journey from molecule to physiology.Channels (Austin). 2013 Nov-Dec;7(6):442-58. doi: 10.4161/chan.26242. Epub 2013 Sep 11. Channels (Austin). 2013. PMID: 24025517 Free PMC article. Review.
Cited by
-
Spontaneous and evoked contractions are regulated by PKC-mediated signaling in detrusor smooth muscle: involvement of BK channels.Am J Physiol Renal Physiol. 2013 Mar 1;304(5):F451-62. doi: 10.1152/ajprenal.00639.2011. Epub 2012 Dec 26. Am J Physiol Renal Physiol. 2013. PMID: 23269650 Free PMC article.
-
Effects of combined treatment of tadalafil and tamsulosin on bladder dysfunction via the inhibition of afferent nerve activities in a rat model of bladder outlet obstruction.Int Urol Nephrol. 2018 May;50(5):839-844. doi: 10.1007/s11255-018-1835-8. Epub 2018 Mar 8. Int Urol Nephrol. 2018. PMID: 29520560
-
Partial bladder outlet obstruction is associated with decreased expression and function of the small-conductance Ca2+-activated K+ channel in guinea pig detrusor smooth muscle.Int Urol Nephrol. 2017 Jan;49(1):17-26. doi: 10.1007/s11255-016-1455-0. Epub 2016 Nov 14. Int Urol Nephrol. 2017. PMID: 27844406
-
Impact of partial urethral obstruction on bladder function: time-dependent changes and functional correlates of altered expression of Ca²⁺ signaling regulators.Am J Physiol Renal Physiol. 2012 Jun 15;302(12):F1517-28. doi: 10.1152/ajprenal.00016.2012. Epub 2012 Mar 21. Am J Physiol Renal Physiol. 2012. PMID: 22442207 Free PMC article.
-
Large-conductance voltage- and Ca2+-activated K+ channels regulate human detrusor smooth muscle function.Am J Physiol Cell Physiol. 2011 Oct;301(4):C903-12. doi: 10.1152/ajpcell.00495.2010. Epub 2011 Jun 22. Am J Physiol Cell Physiol. 2011. PMID: 21697543 Free PMC article.
References
-
- Andersson KE. Storage and voiding symptoms: pathophysiologic aspects. Urology 62: 3–10, 2003 - PubMed
-
- Bonefeld BE, Elfving B, Wegener G. Reference genes for normalization: a study of rat brain tissue. Synapse 62: 302–309, 2008 - PubMed
-
- Braverman AS, Tibb AS, Ruggieri MR., Sr M2 and M3 muscarinic receptor activation of urinary bladder contractile signal transduction. I. Normal rat bladder. J Pharmacol Exp Ther 316: 869–874, 2006 - PubMed
-
- Brenner R, Jegla TJ, Wickenden A, Liu Y, Aldrich RW. Cloning and functional characterization of novel large conductance calcium-activated potassium channel beta subunits, hKCNMB3 and hKCNMB4. J Biol Chem 275: 6453–6461, 2000 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

