Sodium-hydrogen exchanger regulatory factor 1 (NHERF-1) transduces signals that mediate dopamine inhibition of sodium-phosphate co-transport in mouse kidney
- PMID: 20200151
- PMCID: PMC2859505
- DOI: 10.1074/jbc.M109.094359
Sodium-hydrogen exchanger regulatory factor 1 (NHERF-1) transduces signals that mediate dopamine inhibition of sodium-phosphate co-transport in mouse kidney
Abstract
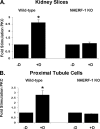
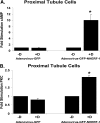
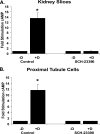
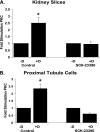
Dopamine inhibited phosphate transport in isolated renal brush border membrane vesicles and in cultured renal proximal tubule cells from wild-type but not from NHERF-1 null mice. Co-immunoprecipitation experiments established that NHERF-1 associated with D1-like receptors. In wild-type mice, dopamine stimulated cAMP accumulation and protein kinase C (PKC) activity in renal proximal tubule cells, an effect that was abolished by SCH-23390, a D1-like receptor antagonist. In NHERF-1 null kidney tissue; however, dopamine failed to stimulate either cAMP accumulation or PKC activity. Infection of proximal tubule cells from NHERF-1 null mice with adenovirus-green fluorescent protein-NHERF-1 restored the ability of dopamine to stimulate cAMP and PKC. Finally, in (32)P-labeled wild-type proximal tubule cells and in opossum kidney cells, dopamine increased NHERF-1 phosphorylation at serine 77 of the PDZ I domain of NHERF-1, a site previously shown to attenuate binding of cellular targets including the Npt2a (sodium-dependent phosphate transporter 2a). Together, these studies establish that NHERF-1 plays a key role in dopamine signaling and is also a downstream target of D1-like receptors in the mouse kidney. These studies suggest a novel role for the PDZ adapter protein NHERF-1 in coordinating dopamine signals that inhibit renal phosphate transport.
Figures
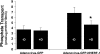
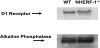
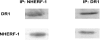



References
-
- Cunningham R., Steplock D., E X., Biswas R. S., Wang F., Shenolikar S., Weinman E. J. (2006) Am. J. Physiol. Renal Physiol. 291, F896–F901 - PubMed
-
- Weinman E. J., Hall R. A., Friedman P. A., Liu-Chen L. Y., Shenolikar S. (2006) Annu. Rev. Physiol. 68, 491–505 - PubMed
-
- Hall R. A., Lefkowitz R. J. (2002) Circ. Res. 91, 672–680 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

