Lysosomal degradation of alpha-synuclein in vivo
- PMID: 20200163
- PMCID: PMC2859524
- DOI: 10.1074/jbc.M109.074617
Lysosomal degradation of alpha-synuclein in vivo
Abstract
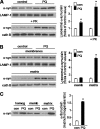
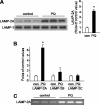
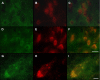
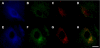
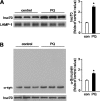
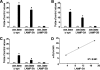
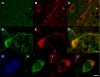
Pathologic accumulation of alpha-synuclein is a feature of human parkinsonism and other neurodegenerative diseases. This accumulation may be counteracted by mechanisms of protein degradation that have been investigated in vitro but remain to be elucidated in animal models. In this study, lysosomal clearance of alpha-synuclein in vivo was indicated by the detection of alpha-synuclein in the lumen of lysosomes isolated from the mouse midbrain. When neuronal alpha-synuclein expression was enhanced as a result of toxic injury (i.e. treatment of mice with the herbicide paraquat) or transgenic protein overexpression, the intralysosomal content of alpha-synuclein was also significantly increased. This effect was paralleled by a marked elevation of the lysosome-associated membrane protein type 2A (LAMP-2A) and the lysosomal heat shock cognate protein of 70 kDa (hsc70), two essential components of chaperone-mediated autophagy (CMA). Immunofluorescence microscopy revealed an increase in punctate (lysosomal) LAMP-2A staining that co-localized with alpha-synuclein within nigral dopaminergic neurons of paraquat-treated and alpha-synuclein-overexpressing animals. The data provide in vivo evidence of lysosomal degradation of alpha-synuclein under normal conditions and, quite importantly, under conditions of enhanced protein burden. In the latter, increased lysosomal clearance of alpha-synuclein was mediated, at least in part, by CMA induction. It is conceivable that these neuronal mechanisms of protein clearance play an important role in neurodegenerative processes characterized by abnormal alpha-synuclein buildup.
Figures



References
-
- Singleton A. B., Farrer M., Johnson J., Singleton A., Hague S., Kachergus J., Hulihan M., Peuralinna T., Dutra A., Nussbaum R., Lincoln S., Crawley A., Hanson M., Maraganore D., Adler C., Cookson M. R., Muenter M., Baptista M., Miller D., Blancato J., Hardy J., Gwinn-Hardy K. (2003) Science 302, 841. - PubMed
-
- Farrer M., Kachergus J., Forno L., Lincoln S., Wang D. S., Hulihan M., Maraganore D., Gwinn-Hardy K., Wszolek Z., Dickson D., Langston J. W. (2004) Ann. Neurol. 55, 174–179 - PubMed
-
- Eslamboli A., Romero-Ramos M., Burger C., Bjorklund T., Muzyczka N., Mandel R. J., Baker H., Ridley R. M., Kirik D. (2007) Brain 130, 799–815 - PubMed
-
- Webb J. L., Ravikumar B., Atkins J., Skepper J. N., Rubinsztein D. C. (2003) J. Biol. Chem. 278, 25009–25013 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

