Quality monitoring of HIV-1-infected and uninfected peripheral blood mononuclear cell samples in a resource-limited setting
- PMID: 20200187
- PMCID: PMC2884426
- DOI: 10.1128/CVI.00492-09
Quality monitoring of HIV-1-infected and uninfected peripheral blood mononuclear cell samples in a resource-limited setting
Abstract
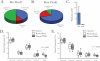
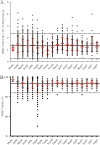
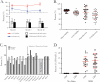
Human immunodeficiency virus type 1 (HIV-1) vaccine and natural history studies are critically dependent on the ability to isolate, cryopreserve, and thaw peripheral blood mononuclear cell (PBMC) samples with a high level of quality and reproducibility. Here we characterize the yield, viability, phenotype, and function of PBMC from HIV-1-infected and uninfected Ugandans and describe measures to ascertain reproducibility and sample quality at the sites that perform cryopreservation. We have developed a comprehensive internal quality control program to monitor processing, including components of method validation. Quality indicators for real-time performance assessment included the time from venipuncture to cryopreservation, time for PBMC processing, yield of PBMC from whole blood, and viability of the PBMC before cryopreservation. Immune phenotype analysis indicated lowered B-cell frequencies following processing and cryopreservation for both HIV-1-infected and uninfected subjects (P < 0.007), but all other major lymphocyte subsets were unchanged. Long-term cryopreservation did not impact function, as unstimulated specimens exhibited low background and all specimens responded to staphylococcal enterotoxin B (SEB) by gamma interferon and interleukin-2 production, as measured by intracellular cytokine staining. Samples stored for more than 3 years did not decay with regard to yield or viability, regardless of HIV-1 infection status. These results demonstrate that it is possible to achieve the high level of quality necessary for vaccine trials and natural history studies in a resource-limited setting and provide strategies for laboratories to monitor PBMC processing performance.
Figures

References
-
- Allsopp, C. E., S. J. Nicholls, and J. Langhorne. 1998. A flow cytometric method to assess antigen-specific proliferative responses of different subpopulations of fresh and cryopreserved human peripheral blood mononuclear cells. J. Immunol. Methods 214:175-186. - PubMed
-
- Appay, V., S. Reynard, V. Voelter, P. Romero, D. E. Speiser, and S. Leyvraz. 2006. Immuno-monitoring of CD8+ T cells in whole blood versus PBMC samples. J. Immunol. Methods 309:192-199. - PubMed
-
- Arroyo, M. A., W. B. Sateren, D. Serwadda, R. H. Gray, M. J. Wawer, N. K. Sewankambo, N. Kiwanuka, G. Kigozi, F. Wabwire-Mangen, M. Eller, L. A. Eller, D. L. Birx, M. L. Robb, and F. E. McCutchan. 2006. Higher HIV-1 incidence and genetic complexity along main roads in Rakai District, Uganda. J. Acquir. Immune Defic. Syndr. 43:440-445. - PubMed
-
- Ashmore, L. M., G. M. Shopp, and B. S. Edwards. 1989. Lymphocyte subset analysis by flow cytometry. Comparison of three different staining techniques and effects of blood storage. J. Immunol. Methods 118:209-215. - PubMed
-
- Baker, C. A., K. McEvers, R. Byaruhanga, R. Mulindwa, D. Atwine, J. Nantiba, N. G. Jones, I. Ssewanyana, and H. Cao. 2008. HIV subtypes induce distinct profiles of HIV-specific CD8(+) T cell responses. AIDS Res. Hum. Retroviruses 24:283-287. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

