Interleukin-7 mediates glucose utilization in lymphocytes through transcriptional regulation of the hexokinase II gene
- PMID: 20200205
- PMCID: PMC2889638
- DOI: 10.1152/ajpcell.00506.2009
Interleukin-7 mediates glucose utilization in lymphocytes through transcriptional regulation of the hexokinase II gene
Abstract
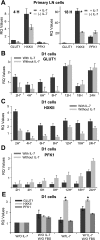
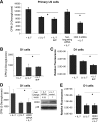
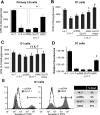
The cytokine interleukin-7 (IL-7) has essential growth activities that maintain the homeostatic balance of the immune system. Little is known of the mechanism by which IL-7 signaling regulates metabolic activity in support of its vital function in lymphocytes. We observed that IL-7 deprivation caused a rapid decline in the metabolism of glucose that was attributable to loss of intracellular glucose retention. To identify the transducer of the IL-7 metabolic signal, we examined the expression of three important regulators of glucose metabolism, the glucose transporter GLUT-1 and two glycolytic enzymes, hexokinase II (HXKII) and phosphofructokinase-1 (PFK-1), using an IL-7-dependent T-cell line and primary lymphocytes. We found that in lymphocytes deprived of IL-7 loss of glucose uptake correlated with decreased expression of HXKII. Readdition of IL-7 to cytokine-deprived lymphocytes restored the transcription of the HXKII gene within 2 h, but not that of GLUT-1 or PFK-1. IL-7-mediated increases in HXKII, but not GLUT-1 or PFK-1, were also observed at the protein level. Inhibition of HXKII with 3-bromopyruvate or specific small-interfering RNA decreased glucose utilization, as well as ATP levels, in the presence of IL-7, whereas overexpression of HXKII, but not GLUT-1, restored glucose retention and increased ATP levels in the absence of IL-7. We conclude that IL-7 controls glucose utilization by regulating the gene expression of HXKII, suggesting a mechanism by which IL-7 supports bioenergetics that control cell fate decisions in lymphocytes.
Figures




Similar articles
-
JunD/AP-1-mediated gene expression promotes lymphocyte growth dependent on interleukin-7 signal transduction.PLoS One. 2012;7(2):e32262. doi: 10.1371/journal.pone.0032262. Epub 2012 Feb 23. PLoS One. 2012. PMID: 22384197 Free PMC article.
-
Glycolytic enzyme hexokinase II is a putative therapeutic target in B-cell malignant lymphoma.Exp Hematol. 2019 Oct;78:46-55.e3. doi: 10.1016/j.exphem.2019.09.023. Epub 2019 Sep 24. Exp Hematol. 2019. PMID: 31560931
-
Studies of gene expression and activity of hexokinase, phosphofructokinase and glycogen synthase in human skeletal muscle in states of altered insulin-stimulated glucose metabolism.Dan Med Bull. 1999 Feb;46(1):13-34. Dan Med Bull. 1999. PMID: 10081651 Review.
-
Glucose metabolism during the final stage of human oocyte maturation: genetic expression of hexokinase, glucose phosphate isomerase and phosphofructokinase.Zygote. 1999 Feb;7(1):45-50. doi: 10.1017/s0967199499000386. Zygote. 1999. PMID: 10216916
-
Follow the ATP: tumor energy production: a perspective.Anticancer Agents Med Chem. 2014;14(9):1187-98. doi: 10.2174/1871520614666140804224637. Anticancer Agents Med Chem. 2014. PMID: 25102360 Review.
Cited by
-
Androgens enhance the glycolytic metabolism and lactate export in prostate cancer cells by modulating the expression of GLUT1, GLUT3, PFK, LDH and MCT4 genes.J Cancer Res Clin Oncol. 2016 Jan;142(1):5-16. doi: 10.1007/s00432-015-1992-4. Epub 2015 Jun 6. J Cancer Res Clin Oncol. 2016. PMID: 26048031 Free PMC article.
-
The major isoforms of Bim contribute to distinct biological activities that govern the processes of autophagy and apoptosis in interleukin-7 dependent lymphocytes.Biochim Biophys Acta. 2012 Oct;1823(10):1877-93. doi: 10.1016/j.bbamcr.2012.06.017. Epub 2012 Jun 21. Biochim Biophys Acta. 2012. PMID: 22728771 Free PMC article.
-
Seeing is believing: illuminating the source of in vivo interleukin-7.Immune Netw. 2011 Feb;11(1):1-10. doi: 10.4110/in.2011.11.1.1. Epub 2011 Feb 28. Immune Netw. 2011. PMID: 21494371 Free PMC article.
-
miR-542-3p Contributes to the HK2-Mediated High Glycolytic Phenotype in Human Glioma Cells.Genes (Basel). 2021 Apr 23;12(5):633. doi: 10.3390/genes12050633. Genes (Basel). 2021. PMID: 33922649 Free PMC article.
-
Non-invasive biomarkers for monitoring the immunotherapeutic response to cancer.J Transl Med. 2020 Dec 9;18(1):471. doi: 10.1186/s12967-020-02656-7. J Transl Med. 2020. PMID: 33298096 Free PMC article. Review.
References
-
- Akbar AN, Borthwick NJ, Wickremasinghe RG, Panayoitidis P, Pilling D, Bofill M, Krajewski S, Reed JC, Salmon M. Interleukin-2 receptor common gamma-chain signaling cytokines regulate activated T cell apoptosis in response to growth factor withdrawal: selective induction of anti-apoptotic (bcl-2, bcl-xL) but not pro-apoptotic (bax, bcl-xS) gene expression. Eur J Immunol 26: 294–299, 1996 - PubMed
-
- Barata JT, Cardoso AA, Boussiotis VA. Interleukin-7 in T-cell acute lymphoblastic leukemia: an extrinsic factor supporting leukemogenesis? Leuk Lymphoma 46: 483–495, 2005 - PubMed
-
- Barthel A, Okino ST, Liao J, Nakatani K, Li J, Whitlock JP, Jr, Roth RA. Regulation of GLUT1 gene transcription by the serine/threonine kinase Akt1. J Biol Chem 274: 20281–20286, 1999 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

