A myosin IK-Abp1-PakB circuit acts as a switch to regulate phagocytosis efficiency
- PMID: 20200225
- PMCID: PMC2861610
- DOI: 10.1091/mbc.e09-06-0485
A myosin IK-Abp1-PakB circuit acts as a switch to regulate phagocytosis efficiency
Abstract
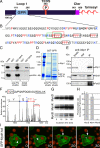
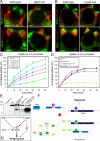
Actin dynamics and myosin (Myo) contractile forces are necessary for formation and closure of the phagocytic cup. In Dictyostelium, the actin-binding protein Abp1 and myosin IK are enriched in the closing cup and especially at an actin-dense constriction furrow formed around the neck of engulfed budded yeasts. This phagocytic furrow consists of concentric overlapping rings of MyoK, Abp1, Arp3, coronin, and myosin II, following an order strikingly reminiscent of the overall organization of the lamellipodium of migrating cells. Mutation analyses of MyoK revealed that both a C-terminal farnesylation membrane anchor and a Gly-Pro-Arg domain that interacts with profilin and Abp1 were necessary for proper localization in the furrow and efficient phagocytosis. Consequently, we measured the binding affinities of these interactions and unraveled further interactions with profilins, dynamin A, and PakB. Due to the redundancy of the interaction network, we hypothesize that MyoK and Abp1 are restricted to regulatory roles and might affect the dynamic of cup progression. Indeed, phagocytic uptake was regulated antagonistically by MyoK and Abp1. MyoK is phosphorylated by PakB and positively regulates phagocytosis, whereas binding of Abp1 negatively regulates PakB and MyoK. We conclude that a MyoK-Abp1-PakB circuit acts as a switch regulating phagocytosis efficiency of large particles.
Figures





Similar articles
-
The balance in the delivery of ER components and the vacuolar proton pump to the phagosome depends on myosin IK in Dictyostelium.Mol Cell Proteomics. 2012 Oct;11(10):886-900. doi: 10.1074/mcp.M112.017608. Epub 2012 Jun 26. Mol Cell Proteomics. 2012. PMID: 22736568 Free PMC article.
-
PakB binds to the SH3 domain of Dictyostelium Abp1 and regulates its effects on cell polarity and early development.Mol Biol Cell. 2013 Jul;24(14):2216-27. doi: 10.1091/mbc.E12-12-0883. Epub 2013 May 22. Mol Biol Cell. 2013. PMID: 23699396 Free PMC article.
-
Cellular distribution and functions of wild-type and constitutively activated Dictyostelium PakB.Mol Biol Cell. 2005 Jan;16(1):238-47. doi: 10.1091/mbc.e04-06-0534. Epub 2004 Oct 27. Mol Biol Cell. 2005. PMID: 15509655 Free PMC article.
-
Dictyostelium myosin IK is involved in the maintenance of cortical tension and affects motility and phagocytosis.J Cell Sci. 2000 Feb;113 ( Pt 4):621-33. doi: 10.1242/jcs.113.4.621. J Cell Sci. 2000. PMID: 10652255
-
Myosin I: from yeast to human.Cell Mol Life Sci. 2008 Jul;65(14):2128-37. doi: 10.1007/s00018-008-7435-5. Cell Mol Life Sci. 2008. PMID: 18344022 Free PMC article. Review.
Cited by
-
Myosin I links PIP3 signaling to remodeling of the actin cytoskeleton in chemotaxis.Sci Signal. 2012 Jan 31;5(209):ra10. doi: 10.1126/scisignal.2002446. Sci Signal. 2012. PMID: 22296834 Free PMC article.
-
Filamentous morphology of bacteria delays the timing of phagosome morphogenesis in macrophages.J Cell Biol. 2013 Dec 23;203(6):1081-97. doi: 10.1083/jcb.201304095. J Cell Biol. 2013. PMID: 24368810 Free PMC article.
-
A plasma membrane template for macropinocytic cups.Elife. 2016 Dec 13;5:e20085. doi: 10.7554/eLife.20085. Elife. 2016. PMID: 27960076 Free PMC article.
-
The balance in the delivery of ER components and the vacuolar proton pump to the phagosome depends on myosin IK in Dictyostelium.Mol Cell Proteomics. 2012 Oct;11(10):886-900. doi: 10.1074/mcp.M112.017608. Epub 2012 Jun 26. Mol Cell Proteomics. 2012. PMID: 22736568 Free PMC article.
-
Eat Prey, Live: Dictyostelium discoideum As a Model for Cell-Autonomous Defenses.Front Immunol. 2018 Jan 4;8:1906. doi: 10.3389/fimmu.2017.01906. eCollection 2017. Front Immunol. 2018. PMID: 29354124 Free PMC article. Review.
References
-
- Bertling E., Quintero-Monzon O., Mattila P. K., Goode B. L., Lappalainen P. Mechanism and biological role of profilin-Srv2/CAP interaction. J. Cell Sci. 2007;120:1225–1234. - PubMed
-
- Clarke M., Maddera L. Phagocyte meets prey: uptake, internalization, and killing of bacteria by Dictyostelium amoebae. Eur. J. Cell Biol. 2006;85:1001–1010. - PubMed
-
- Crawley S. W., de la Roche M. A., Lee S. F., Li Z., Chitayat S., Smith S. P., Cote G. P. Identification and characterization of an 8-kDa light chain associated with Dictyostelium discoideum MyoB, a class I myosin. J. Biol. Chem. 2006;281:6307–6315. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

