Welcome to a new kind of tension: translating kinetochore mechanics into a wait-anaphase signal
- PMID: 20200228
- PMCID: PMC2831757
- DOI: 10.1242/jcs.064790
Welcome to a new kind of tension: translating kinetochore mechanics into a wait-anaphase signal
Abstract
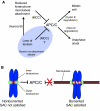
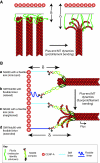
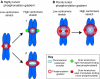
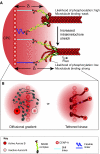
Recent high-resolution studies of kinetochore structure have transformed the way researchers think about this crucial macro-molecular complex, which is essential for ensuring chromosome segregation occurs faithfully during cell division. Kinetochores mediate the interaction between chromosomes and the plus-ends of dynamic spindle microtubules and control the timing of anaphase onset by regulating the spindle assembly checkpoint (SAC). There is much debate in the SAC research community as to whether mitotic cells sense only microtubule attachment at the kinetochore, or both attachment and tension, before committing to anaphase. In this Commentary, we present a brief history of the tension-versus-attachment debate, summarize recent advances in our understanding of kinetochore structure and focus on the implications of a phenomenon known as intrakinetochore stretch for SAC regulation. We also hypothesize how intrakinetochore stretch might impact SAC function by regulating both microtubule attachment stability and the localization and activity of checkpoint components at the kinetochore.
Figures

Similar articles
-
CELL DIVISION CYCLE. Competition between MPS1 and microtubules at kinetochores regulates spindle checkpoint signaling.Science. 2015 Jun 12;348(6240):1264-7. doi: 10.1126/science.aaa4055. Epub 2015 Jun 11. Science. 2015. PMID: 26068855
-
BUB-1 promotes amphitelic chromosome biorientation via multiple activities at the kinetochore.Elife. 2018 Dec 14;7:e40690. doi: 10.7554/eLife.40690. Elife. 2018. PMID: 30547880 Free PMC article.
-
Spindle checkpoint proteins and chromosome-microtubule attachment in budding yeast.J Cell Biol. 2004 Feb 16;164(4):535-46. doi: 10.1083/jcb.200308100. Epub 2004 Feb 9. J Cell Biol. 2004. PMID: 14769859 Free PMC article.
-
Attachment issues: kinetochore transformations and spindle checkpoint silencing.Curr Opin Cell Biol. 2016 Apr;39:101-8. doi: 10.1016/j.ceb.2016.02.016. Epub 2016 Mar 3. Curr Opin Cell Biol. 2016. PMID: 26947988 Review.
-
How the SAC gets the axe: Integrating kinetochore microtubule attachments with spindle assembly checkpoint signaling.Bioarchitecture. 2015;5(1-2):1-12. doi: 10.1080/19490992.2015.1090669. Epub 2015 Oct 2. Bioarchitecture. 2015. PMID: 26430805 Free PMC article. Review.
Cited by
-
Staging a recovery from mitotic arrest: Unusual ways of Cdk1.Bioarchitecture. 2012 Feb 1;2(2):33-37. doi: 10.4161/bioa.20421. Bioarchitecture. 2012. PMID: 22754627 Free PMC article.
-
The chromosomal passenger complex (CPC): from easy rider to the godfather of mitosis.Nat Rev Mol Cell Biol. 2012 Dec;13(12):789-803. doi: 10.1038/nrm3474. Nat Rev Mol Cell Biol. 2012. PMID: 23175282 Free PMC article. Review.
-
Low tension recruits the yeast Aurora B protein Ipl1 to centromeres in metaphase.J Cell Sci. 2023 Aug 15;136(16):jcs261416. doi: 10.1242/jcs.261416. Epub 2023 Aug 17. J Cell Sci. 2023. PMID: 37519149 Free PMC article.
-
Monitoring spindle orientation: Spindle position checkpoint in charge.Cell Div. 2010 Dec 11;5:28. doi: 10.1186/1747-1028-5-28. Cell Div. 2010. PMID: 21143992 Free PMC article.
-
Understanding the structural basis for controlling chromosome division.Philos Trans A Math Phys Eng Sci. 2015 Mar 6;373(2036):20130392. doi: 10.1098/rsta.2013.0392. Philos Trans A Math Phys Eng Sci. 2015. PMID: 25624511 Free PMC article. Review.
References
-
- Ahonen L. J., Kallio M. J., Daum J. R., Bolton M., Manke I. A., Yaffe M. B., Stukenberg P. T., Gorbsky G. J. (2005). Polo-like kinase 1 creates the tension-sensing 3F3/2 phosphoepitope and modulates the association of spindle-checkpoint proteins at kinetochores. Curr. Biol. 15, 1078-1089 - PubMed
-
- Andrews P. D., Ovechkina Y., Morrice N., Wagenbach M., Duncan K., Wordeman L., Swedlow J. R. (2004). Aurora B regulates MCAK at the mitotic centromere. Dev. Cell 6, 253-268 - PubMed
-
- Basto R., Gomes R., Karess R. E. (2000). Rough deal and Zw10 are required for the metaphase checkpoint in Drosophila. Nat. Cell Biol. 2, 939-943 - PubMed
-
- Beardmore V. A., Ahonen L. J., Gorbsky G. J., Kallio M. J. (2004). Survivin dynamics increases at centromeres during G2/M phase transition and is regulated by microtubule-attachment and Aurora B kinase activity. J. Cell Sci. 117, 4033-4042 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

