A role for the C terminus of Mopeia virus nucleoprotein in its incorporation into Z protein-induced virus-like particles
- PMID: 20200234
- PMCID: PMC2863806
- DOI: 10.1128/JVI.02417-09
A role for the C terminus of Mopeia virus nucleoprotein in its incorporation into Z protein-induced virus-like particles
Abstract
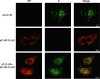
Arenaviruses are enveloped, negative-strand RNA viruses. For several arenaviruses, virus-like particle (VLP) formation requires the viral matrix Z protein. However, the mechanism by which viral ribonucleoprotein complexes are incorporated into virions is poorly understood. Here, we show that the expression of the Z protein and nucleoprotein (NP) of Mopeia virus, a close relative of the pathogenic Lassa virus, resulted in the highly selective incorporation of the NP protein into Z protein-induced VLPs. Moreover, the Z protein promoted the association of NP with cellular membranes, suggesting that the association of NP, Z, and the cellular membranes may facilitate the efficient incorporation of NP into VLPs. By employing a series of NP deletion constructs and testing their VLP incorporation, we further demonstrated an important role for the C-terminal half of NP in its incorporation into VLPs.
Figures




References
-
- Briese, T., J. T. Paweska, L. K. McMullan, S. K. Hutchison, C. Street, G. Palacios, M. L. Khristova, J. Weyer, R. Swanepoel, M. Egholm, S. T. Nichol, and W. I. Lipkin. 2009. Genetic detection and characterization of Lujo virus, a new hemorrhagic fever-associated arenavirus from southern Africa. PLoS Pathog. 5:e1000455. - PMC - PubMed
-
- Buchmeier, M. J., J. C. de la Torre, and C. J. Peters. 2007. Arenaviridae, p. 1635-1668. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
-
- Burns, J. W., and M. J. Buchmeier. 1991. Protein-protein interactions in lymphocytic choriomeningitis virus. Virology 183:620-629. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

