Analysis of apoptosis of memory T cells and dendritic cells during the early stages of viral infection or exposure to toll-like receptor agonists
- PMID: 20200235
- PMCID: PMC2863811
- DOI: 10.1128/JVI.02571-09
Analysis of apoptosis of memory T cells and dendritic cells during the early stages of viral infection or exposure to toll-like receptor agonists
Abstract
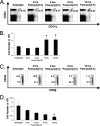
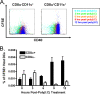
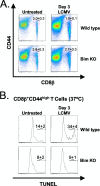
Profound type I interferon (IFN-I)-dependent attrition of memory CD8 and CD4 T cells occurs early during many infections. It is dramatic at 2 to 4 days following lymphocytic choriomeningitis virus (LCMV) infection of mice and can be elicited by the IFN-inducing Toll receptor agonist poly(I:C). We show that this attrition occurs in many organs, indicating that it is due to T cell loss rather than redistribution. This loss correlated with elevated intracellular staining of T cells ex vivo for activated caspases but with only low levels of ex vivo staining with annexin V, probably due to the rapid clearance of apoptotic cells in vivo. Instead, a high frequency of annexin V-reactive CD8alpha(+) dendritic cells (DCs), which are known to be highly phagocytic, accumulated in the spleen as the memory T cell populations disappeared. After short in vitro incubation, memory phenotype T cells isolated from LCMV-infected mice (day 3) or mice treated with poly(I:C) (12 h) displayed substantial DNA fragmentation, as detected by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay, compared to T cells isolated from uninfected mice, indicating a role for apoptosis in the memory T cell attrition. This apoptosis of memory CD8 T cells early during LCMV infection was reduced in mice lacking the proapoptotic molecule Bim. Evidence is presented showing that high levels of T cell attrition, as found in young mice, correlate with reduced immunodomination by cross-reactive memory cells.
Figures





Similar articles
-
IFN-induced attrition of CD8 T cells in the presence or absence of cognate antigen during the early stages of viral infections.J Immunol. 2006 Apr 1;176(7):4284-95. doi: 10.4049/jimmunol.176.7.4284. J Immunol. 2006. PMID: 16547266
-
Attrition of bystander CD8 T cells during virus-induced T-cell and interferon responses.J Virol. 2001 Jul;75(13):5965-76. doi: 10.1128/JVI.75.13.5965-5976.2001. J Virol. 2001. PMID: 11390598 Free PMC article.
-
Stability of virus-specific CD4+ T cell frequencies from acute infection into long term memory.J Immunol. 1998 Jul 1;161(1):367-74. J Immunol. 1998. PMID: 9647245
-
Adoptive immunotherapy induces CNS dendritic cell recruitment and antigen presentation during clearance of a persistent viral infection.J Exp Med. 2006 Aug 7;203(8):1963-75. doi: 10.1084/jem.20060039. Epub 2006 Jul 17. J Exp Med. 2006. PMID: 16847068 Free PMC article.
-
Viruses and T cell turnover: evidence for bystander proliferation.Immunol Rev. 1996 Apr;150:129-42. doi: 10.1111/j.1600-065x.1996.tb00699.x. Immunol Rev. 1996. PMID: 8782705 Review. No abstract available.
Cited by
-
Type 1 interferons and antiviral CD8 T-cell responses.PLoS Pathog. 2012 Jan;8(1):e1002352. doi: 10.1371/journal.ppat.1002352. Epub 2012 Jan 5. PLoS Pathog. 2012. PMID: 22241987 Free PMC article. Review. No abstract available.
-
Placenta-specific 8 limits IFNγ production by CD4 T cells in vitro and promotes establishment of influenza-specific CD8 T cells in vivo.PLoS One. 2020 Jul 8;15(7):e0235706. doi: 10.1371/journal.pone.0235706. eCollection 2020. PLoS One. 2020. PMID: 32639988 Free PMC article.
-
Vaccination and heterologous immunity: educating the immune system.Trans R Soc Trop Med Hyg. 2015 Jan;109(1):62-9. doi: 10.1093/trstmh/tru198. Trans R Soc Trop Med Hyg. 2015. PMID: 25573110 Free PMC article. Review.
-
Disease-promoting effects of type I interferons in viral, bacterial, and coinfections.J Interferon Cytokine Res. 2015 Apr;35(4):252-64. doi: 10.1089/jir.2014.0227. Epub 2015 Feb 25. J Interferon Cytokine Res. 2015. PMID: 25714109 Free PMC article. Review.
-
Exposure to the viral by-product dsRNA or Coxsackievirus B5 triggers pancreatic beta cell apoptosis via a Bim / Mcl-1 imbalance.PLoS Pathog. 2011 Sep;7(9):e1002267. doi: 10.1371/journal.ppat.1002267. Epub 2011 Sep 22. PLoS Pathog. 2011. PMID: 21977009 Free PMC article.
References
-
- Albert, M. L., B. Sauter, and N. Bhardwaj. 1998. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 392:86-89. - PubMed
-
- Bahl, K., S. K. Kim, C. Calcagno, D. Ghersi, R. Puzone, F. Celada, L. K. Selin, and R. M. Welsh. 2006. IFN-induced attrition of CD8 T cells in the presence or absence of cognate antigen during the early stages of viral infections. J. Immunol. 176:4284-4295. - PubMed
-
- Banchereau, J., F. Bazan, D. Blanchard, F. Briere, J. P. Galizzi, C. van Kooten, Y. J. Liu, F. Rousset, and S. Saeland. 1994. The CD40 antigen and its ligand. Annu. Rev. Immunol. 12:881-922. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI081675/AI/NIAID NIH HHS/United States
- R01 AI054455/AI/NIAID NIH HHS/United States
- P01 DK080665/DK/NIDDK NIH HHS/United States
- AI073871/AI/NIAID NIH HHS/United States
- R01 NS054948/NS/NINDS NIH HHS/United States
- U01 AI073871/AI/NIAID NIH HHS/United States
- R01 CA065861/CA/NCI NIH HHS/United States
- AI017672/AI/NIAID NIH HHS/United States
- NS054948/NS/NINDS NIH HHS/United States
- DK080665/DK/NIDDK NIH HHS/United States
- CA65861/CA/NCI NIH HHS/United States
- R37 AI017672/AI/NIAID NIH HHS/United States
- Howard Hughes Medical Institute/United States
- AI46692/AI/NIAID NIH HHS/United States
- AI054455/AI/NIAID NIH HHS/United States
- AI081675/AI/NIAID NIH HHS/United States
- R01 AI017672/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

