Innate immune responses and permissiveness to ranavirus infection of peritoneal leukocytes in the frog Xenopus laevis
- PMID: 20200236
- PMCID: PMC2863837
- DOI: 10.1128/JVI.02486-09
Innate immune responses and permissiveness to ranavirus infection of peritoneal leukocytes in the frog Xenopus laevis
Abstract
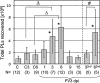
Ranaviruses such as frog virus 3 ([FV3] family Iridoviridae) are increasingly prevalent pathogens that infect reptiles, amphibians, and fish worldwide. Whereas studies in the frog Xenopus laevis have revealed the critical involvement of CD8 T-cell and antibody responses in host resistance to FV3, little is known about the role played by innate immunity to infection with this virus. We have investigated the occurrence, composition, activation status, and permissiveness to infection of peritoneal leukocytes (PLs) in Xenopus adults during FV3 infection by microscopy, flow cytometry, and reverse transcription-PCR. The total number of PLs and the relative fraction of activated mononucleated macrophage-like cells significantly increase as early as 1 day postinfection (dpi), followed by NK cells at 3 dpi, before the peak of the T-cell response at 6 dpi. FV3 infection also induces a rapid upregulation of proinflammatory genes including arginase 1, interleukin-1beta, and tumor necrosis factor alpha. Although PLs are susceptible to FV3 infection, as evidenced by apoptotic cells, active FV3 transcription, and the detection of viral particles by electron microscopy, the infection is weaker (fewer infectious particles), more transitory, and involves a smaller fraction (less than 1%) of PLs than the kidney, the main site of infection. However, viral DNA remains detectable in PLs for at least 3 weeks postinfection, past the point of viral clearance observed in the kidneys. This suggests that although PLs are actively involved in anti-FV3 immune responses, some of these cells can be permissive and harbor quiescent, asymptomatic FV3.
Figures


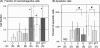




References
-
- Chinchar, V. G. 2002. Ranaviruses (family Iridoviridae): emerging cold-blooded killers. Arch. Virol. 147:447-470. - PubMed
-
- Chinchar, V. G., L. Bryan, J. Wang, S. Long, and G. D. Chinchar. 2003. Induction of apoptosis in frog virus 3-infected cells. Virology 306:303-312. - PubMed
-
- Chinchar, V. G., D. W. Metzger, A. Granoff, and R. Goorha. 1984. Localization of frog virus 3 proteins using monoclonal antibodies. Virology 137:211-216. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

