Monocytes regulate T cell migration through the glia limitans during acute viral encephalitis
- PMID: 20200240
- PMCID: PMC2863800
- DOI: 10.1128/JVI.00051-10
Monocytes regulate T cell migration through the glia limitans during acute viral encephalitis
Abstract
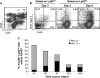
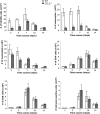
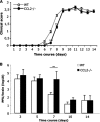
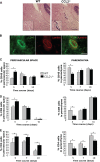
Leukocyte access into the central nervous system (CNS) parenchyma is tightly regulated by the blood-brain barrier (BBB). Leukocyte migration through the endothelial cell wall into the perivascular space is well characterized; however, mechanisms regulating their penetration through the glia limitans into the parenchyma are less well studied, and the role of monocytes relative to neutrophils is poorly defined. Acute viral encephalitis was thus induced in CCL2-deficient (CCL2(-/-)) mice to specifically abrogate monocyte recruitment. Impaired monocyte recruitment prolonged T cell retention in the perivascular space, although no difference in overall CNS accumulation of CD4 or CD8 T cells was detected by flow cytometry. Delayed penetration to the CNS parenchyma was not associated with reduced or altered expression of either matrix metalloproteinases (MMP) or the T cell chemoattractants CXCL10 and CCL5. Nevertheless, decreased parenchymal leukocyte infiltration delayed T cell-mediated control of virus replication as well as clinical disease. These data are the first to demonstrate that the rapid monocyte recruitment into the CNS during viral encephalitis is dispensable for T cell migration across the blood vessel endothelium. However, monocytes facilitate penetration through the glia limitans. Thus, the rapid monocyte response to viral encephalitis constitutes an indirect antiviral pathway by aiding access of effector T cells to the site of viral infection.
Figures

Similar articles
-
MMP-independent role of TIMP-1 at the blood brain barrier during viral encephalomyelitis.ASN Neuro. 2013 Nov 26;5(5):e00127. doi: 10.1042/AN20130033. ASN Neuro. 2013. PMID: 24156369 Free PMC article.
-
Differential Roles of Chemokines CCL2 and CCL7 in Monocytosis and Leukocyte Migration during West Nile Virus Infection.J Immunol. 2015 Nov 1;195(9):4306-18. doi: 10.4049/jimmunol.1500352. Epub 2015 Sep 23. J Immunol. 2015. PMID: 26401006 Free PMC article.
-
A model for monocyte migration through the blood-brain barrier during HIV-1 encephalitis.J Immunol. 1997 Apr 1;158(7):3499-510. J Immunol. 1997. PMID: 9120312
-
Capture, crawl, cross: the T cell code to breach the blood-brain barriers.Trends Immunol. 2012 Dec;33(12):579-89. doi: 10.1016/j.it.2012.07.004. Epub 2012 Aug 25. Trends Immunol. 2012. PMID: 22926201 Review.
-
Perivascular spaces and the two steps to neuroinflammation.J Neuropathol Exp Neurol. 2008 Dec;67(12):1113-21. doi: 10.1097/NEN.0b013e31818f9ca8. J Neuropathol Exp Neurol. 2008. PMID: 19018243 Review.
Cited by
-
The chemokine receptor CXCR2 and coronavirus-induced neurologic disease.Virology. 2013 Jan 5;435(1):110-7. doi: 10.1016/j.virol.2012.08.049. Virology. 2013. PMID: 23217621 Free PMC article. Review.
-
Myd88 Initiates Early Innate Immune Responses and Promotes CD4 T Cells during Coronavirus Encephalomyelitis.J Virol. 2015 Sep;89(18):9299-312. doi: 10.1128/JVI.01199-15. Epub 2015 Jul 1. J Virol. 2015. PMID: 26136579 Free PMC article.
-
Differential Regulation of Self-reactive CD4+ T Cells in Cervical Lymph Nodes and Central Nervous System during Viral Encephalomyelitis.Front Immunol. 2016 Sep 21;7:370. doi: 10.3389/fimmu.2016.00370. eCollection 2016. Front Immunol. 2016. PMID: 27708643 Free PMC article.
-
ELR(+) chemokine signaling in host defense and disease in a viral model of central nervous system disease.Front Cell Neurosci. 2014 Jun 17;8:165. doi: 10.3389/fncel.2014.00165. eCollection 2014. Front Cell Neurosci. 2014. PMID: 24987333 Free PMC article. Review.
-
Promoting remyelination: utilizing a viral model of demyelination to assess cell-based therapies.Expert Rev Neurother. 2014 Oct;14(10):1169-79. doi: 10.1586/14737175.2014.955854. Expert Rev Neurother. 2014. PMID: 25245576 Free PMC article. Review.
References
-
- Agrawal, S., P. Anderson, M. Durbeej, N. van Rooijen, F. Ivars, G. Opdenakker, and L. M. Sorokin. 2006. Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation in experimental autoimmune encephalomyelitis. J. Exp. Med. 203:1007-1019. - PMC - PubMed
-
- Bar-Or, A., R. K. Nuttall, M. Duddy, A. Alter, H. J. Kim, I. Ifergan, C. J. Pennington, P. Bourgoin, D. R. Edwards, and V. W. Yong. 2003. Analyses of all matrix metalloproteinase members in leukocytes emphasize monocytes as major inflammatory mediators in multiple sclerosis. Brain 126:2738-2749. - PubMed
-
- Bartholomaus, I., N. Kawakami, F. Odoardi, C. Schlager, D. Miljkovic, J. W. Ellwart, W. E. Klinkert, C. Flugel-Koch, T. B. Issekutz, H. Wekerle, and A. Flugel. 2009. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature 462:94-98. - PubMed
-
- Bechmann, I., I. Galea, and V. H. Perry. 2007. What is the blood-brain barrier (not)? Trends Immunol. 28:5-11. - PubMed
-
- Bergmann, C. C., J. D. Altman, D. Hinton, and S. A. Stohlman. 1999. Inverted immunodominance and impaired cytolytic function of CD8+ T cells during viral persistence in the central nervous system. J. Immunol. 163:3379-3387. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

