Binding site on the transferrin receptor for the parvovirus capsid and effects of altered affinity on cell uptake and infection
- PMID: 20200243
- PMCID: PMC2863798
- DOI: 10.1128/JVI.02623-09
Binding site on the transferrin receptor for the parvovirus capsid and effects of altered affinity on cell uptake and infection
Abstract
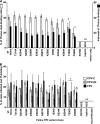
Canine parvovirus (CPV) and its relative feline panleukopenia virus (FPV) bind the transferrin receptor type 1 (TfR) to infect their host cells but show differences in the interactions with the feline and canine TfRs that determine viral host range and tissue tropism. We changed apical and protease-like domain residues by introducing point mutations and adding or removing glycosylation signals, and we then examined the interactions of those mutant TfRs with the capsids. Most substitutions had little effect on virus binding and uptake. However, mutations of several sites in the apical domain of the receptor either prevented binding to the capsids or reduced the affinity of receptor binding to various degrees. Glycans within the virus binding face of the apical domain also controlled capsid binding. CPV, but not the related feline parvovirus, could use receptors containing a canine TfR-specific glycosylation to mediate efficient infection, while addition of other N-linked glycosylation sites into the virus binding face of the feline apical domain reduced or eliminated both binding and infection. Replacement of critical feline TfR residue 221 with every amino acid had effects on binding and infection which were significantly associated with the biochemical properties of the residue replaced. Receptors with reduced affinities mostly showed proportional changes in their ability to mediate infection. Testing feline TfR variants for their binding and uptake patterns in cells showed that low-affinity versions bound fewer capsids and also differed in attachment to the cell surface and filopodia, but transport to the perinuclear endosome was similar.
Figures


References
-
- Agbandje, M., R. McKenna, M. G. Rossmann, M. L. Strassheim, and C. R. Parrish. 1993. Structure determination of feline panleukopenia virus empty particles. Proteins 16:155-171. - PubMed
-
- Arnold, E., and M. G. Rossmann. 1990. Analysis of the structure of a common cold virus, human rhinovirus 14, refined at a resolution of 3.0 Å. J. Mol. Biol. 211:763-801. - PubMed
-
- Bates, G. W., and M. R. Schlabach. 1973. The reaction of ferric salts with transferrin. J. Biol. Chem. 248:3228-3232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

