Phenotypic and functional profile of HIV-inhibitory CD8 T cells elicited by natural infection and heterologous prime/boost vaccination
- PMID: 20200250
- PMCID: PMC2863846
- DOI: 10.1128/JVI.00138-10
Phenotypic and functional profile of HIV-inhibitory CD8 T cells elicited by natural infection and heterologous prime/boost vaccination
Abstract
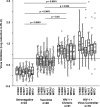
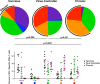
Control of HIV-1 replication following nonsterilizing HIV-1 vaccination could be achieved by vaccine-elicited CD8(+) T-cell-mediated antiviral activity. To date, neither the functional nor the phenotypic profiles of CD8(+) T cells capable of this activity are clearly understood; consequently, little is known regarding the ability of vaccine strategies to elicit them. We used multiparameter flow cytometry and viable cell sorts from phenotypically defined CD8(+) T-cell subsets in combination with a highly standardized virus inhibition assay to evaluate CD8(+) T-cell-mediated inhibition of viral replication. Here we show that vaccination against HIV-1 Env and Gag-Pol by DNA priming followed by recombinant adenovirus type 5 (rAd5) boosting elicited CD8(+) T-cell-mediated antiviral activity against several viruses with either lab-adapted or transmitted virus envelopes. As it did for chronically infected virus controllers, this activity correlated with HIV-1-specific CD107a or macrophage inflammatory protein 1beta (MIP-1beta) expression from HIV-1-specific T cells. Moreover, for vaccinees or virus controllers, purified memory CD8(+) T cells from a wide range of differentiation stages were capable of significantly inhibiting virus replication. Our data define attributes of an antiviral CD8(+) T-cell response that may be optimized in the search for an efficacious HIV-1 vaccine.
Figures


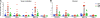
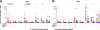
References
-
- Altfeld, M., M. M. Addo, E. S. Rosenberg, F. M. Hecht, P. K. Lee, M. Vogel, X. G. Yu, R. Draenert, M. N. Johnston, D. Strick, T. M. Allen, M. E. Feeney, J. O. Kahn, R. P. Sekaly, J. A. Levy, J. K. Rockstroh, P. J. Goulder, and B. D. Walker. 2003. Influence of HLA-B57 on clinical presentation and viral control during acute HIV-1 infection. AIDS 17:2581-2591. - PubMed
-
- Buchbinder, S. P., D. V. Mehrotra, A. Duerr, D. W. Fitzgerald, R. Mogg, D. Li, P. B. Gilbert, J. R. Lama, M. Marmor, C. Del Rio, M. J. McElrath, D. R. Casimiro, K. M. Gottesdiener, J. A. Chodakewitz, L. Corey, and M. N. Robertson. 2008. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372:1881-1893. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

