Carbohydrate oxidation acidifies endosomes, regulating antigen processing and TLR9 signaling
- PMID: 20200279
- PMCID: PMC2902972
- DOI: 10.4049/jimmunol.0903168
Carbohydrate oxidation acidifies endosomes, regulating antigen processing and TLR9 signaling
Abstract
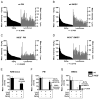
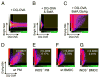
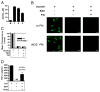
Phagocytes kill encapsulated microbes through oxidative cleavage of surface carbohydrates, releasing glycan fragments and microbial contents that serve as ligands for immune receptors, which tailor the immune response against the offending pathogen. The glycan fragments serve as MHC class II (MHC II) ligands and innate receptor agonists, whereas microbial proteins serve as substrates for proteolytic cleavage and MHC II presentation, and released nucleic acids activate innate pattern-recognition receptors (e.g., TLR9). In the current study, confocal microscopy of live macrophages and dendritic cells revealed that endocytosis of carbohydrates lead to vesicular acidification independent of proton pump activity. Acidification was dependent on NO-mediated oxidation in the presence of the ingested carbohydrate and was sufficient to negatively regulate T cell-dependent polysaccharide Ag cleavage, promote acid-dependent protein Ag processing, and facilitate CpG-mediated TLR9 signaling. Our findings lead to a model in which oxidation of carbohydrates from encapsulated microbes facilitates adaptive immune responses against microbial protein and carbohydrate Ags through promoting Ag processing for MHC II-mediated presentation as well as innate responses against released microbial DNA via TLR9 signaling.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures




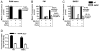

Similar articles
-
Activation of human plasmacytoid dendritic cells by TLR9 impairs Fc gammaRII-mediated uptake of immune complexes and presentation by MHC class II.J Immunol. 2008 Oct 15;181(8):5219-24. doi: 10.4049/jimmunol.181.8.5219. J Immunol. 2008. PMID: 18832675
-
The Toll-like receptor 9 signalling pathway regulates MR1-mediated bacterial antigen presentation in B cells.Immunology. 2017 Oct;152(2):232-242. doi: 10.1111/imm.12759. Epub 2017 Jun 29. Immunology. 2017. PMID: 28518215 Free PMC article.
-
Characteristics of carbohydrate antigen binding to the presentation protein HLA-DR.Glycobiology. 2008 Sep;18(9):707-18. doi: 10.1093/glycob/cwn050. Epub 2008 Jun 4. Glycobiology. 2008. PMID: 18525076 Free PMC article.
-
Toll-like receptor 9 processing: the key event in Toll-like receptor 9 activation?Immunol Lett. 2013 Jan;149(1-2):85-7. doi: 10.1016/j.imlet.2012.11.003. Epub 2012 Nov 24. Immunol Lett. 2013. PMID: 23183093 Review.
-
Bacterial antigen delivery systems: phagocytic processing of bacterial antigens for MHC-I and MHC-II presentation to T cells.Behring Inst Mitt. 1997 Feb;(98):197-211. Behring Inst Mitt. 1997. PMID: 9382741 Review.
Cited by
-
Oxidative depolymerization of polysaccharides by reactive oxygen/nitrogen species.Glycobiology. 2011 Apr;21(4):401-9. doi: 10.1093/glycob/cwq171. Epub 2010 Oct 28. Glycobiology. 2011. PMID: 21030538 Free PMC article. Review.
-
TLR9 is actively recruited to Aspergillus fumigatus phagosomes and requires the N-terminal proteolytic cleavage domain for proper intracellular trafficking.J Immunol. 2010 Dec 15;185(12):7614-22. doi: 10.4049/jimmunol.1002760. Epub 2010 Nov 8. J Immunol. 2010. PMID: 21059889 Free PMC article.
-
M2-Like Macrophages Exhibit Sialic Acid-Enhanced Efferocytosis via the Siglec CD22.FASEB J. 2025 Jul 15;39(13):e70767. doi: 10.1096/fj.202500146RR. FASEB J. 2025. PMID: 40577069
-
The Selenoprotein MsrB1 Instructs Dendritic Cells to Induce T-Helper 1 Immune Responses.Antioxidants (Basel). 2020 Oct 20;9(10):1021. doi: 10.3390/antiox9101021. Antioxidants (Basel). 2020. PMID: 33092166 Free PMC article.
-
Adaptive immune defects against glycoantigens in chronic granulomatous disease via dysregulated nitric oxide production.Eur J Immunol. 2011 Sep;41(9):2562-72. doi: 10.1002/eji.201141396. Epub 2011 Aug 4. Eur J Immunol. 2011. PMID: 21630251 Free PMC article.
References
-
- Cobb BA, Kasper DL. Zwitterionic capsular polysaccharides: the new MHC II-dependent antigens. Cell Microbiol. 2005;7:1398–1403. - PubMed
-
- Brubaker JO, Li Q, Tzianabos AO, Kasper DL, Finberg RW. Mitogenic activity of purified capsular polysaccharide A from Bacteroides fragilis: differential stimulatory effect on mouse and rat lymphocytes in vitro. J Immunol. 1999;162:2235–2242. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

