High-mobility group box-1 protein promotes angiogenesis after peripheral ischemia in diabetic mice through a VEGF-dependent mechanism
- PMID: 20200317
- PMCID: PMC2874711
- DOI: 10.2337/db09-1507
High-mobility group box-1 protein promotes angiogenesis after peripheral ischemia in diabetic mice through a VEGF-dependent mechanism
Abstract
Objective: High-mobility group box-1 (HMGB1) protein is a nuclear DNA-binding protein released from necrotic cells, inducing inflammatory responses and promoting tissue repair and angiogenesis. Diabetic human and mouse tissues contain lower levels of HMGB1 than their normoglycemic counterparts. Deficient angiogenesis after ischemia contributes to worse outcomes of peripheral arterial disease in patients with diabetes. To test the hypothesis that HMGB1 enhances ischemia-induced angiogenesis in diabetes, we administered HMGB1 protein in a mouse hind limb ischemia model using diabetic mice.
Research design and methods: After the induction of diabetes by streptozotocin, we studied ischemia-induced neovascularization in the ischemic hind limb of normoglycemic, diabetic, and HMGB1-treated diabetic mice.
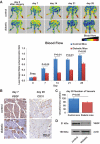
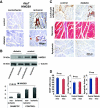
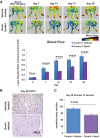
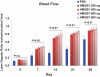
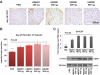
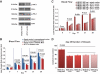
Results: We found that the perfusion recovery was significantly attenuated in diabetic mice compared with normoglycemic control mice. Interestingly, HMGB1 protein expression was lower in the ischemic tissue of diabetic mice than in normoglycemic mice. Furthermore, we observed that HMGB1 administration restored the blood flow recovery and capillary density in the ischemic muscle of diabetic mice, that this process was associated with the increased expression of vascular endothelial growth factor (VEGF), and that HMGB1-induced angiogenesis was significantly reduced by inhibiting VEGF activity.
Conclusions: The results of this study show that endogenous HMGB1 is crucial for ischemia-induced angiogenesis in diabetic mice and that HMGB1 protein administration enhances collateral blood flow in the ischemic hind limbs of diabetic mice through a VEGF-dependent mechanism.
Figures
Comment in
-
Comment on: Biscetti et al. (2010) High-mobility group box-1 protein promotes angiogenesis after peripheral ischemia in diabetic mice through a VEGF-dependent mechanism. Diabetes;59:1496-1505.Diabetes. 2010 Jul;59(7):e7; author reply e8. doi: 10.2337/db10-0445. Diabetes. 2010. PMID: 20587797 No abstract available.
Similar articles
-
Platelet-derived growth factor C promotes revascularization in ischemic limbs of diabetic mice.J Vasc Surg. 2014 May;59(5):1402-9.e1-4. doi: 10.1016/j.jvs.2013.04.053. Epub 2013 Jul 13. J Vasc Surg. 2014. PMID: 23856609
-
Comment on: Biscetti et al. (2010) High-mobility group box-1 protein promotes angiogenesis after peripheral ischemia in diabetic mice through a VEGF-dependent mechanism. Diabetes;59:1496-1505.Diabetes. 2010 Jul;59(7):e7; author reply e8. doi: 10.2337/db10-0445. Diabetes. 2010. PMID: 20587797 No abstract available.
-
Impaired angiogenesis after hindlimb ischemia in type 2 diabetes mellitus: differential regulation of vascular endothelial growth factor receptor 1 and soluble vascular endothelial growth factor receptor 1.Circ Res. 2007 Oct 26;101(9):948-56. doi: 10.1161/CIRCRESAHA.107.160630. Epub 2007 Sep 6. Circ Res. 2007. PMID: 17823371
-
Therapeutic potential of high mobility group box-1 in ischemic injury and tissue regeneration.Curr Vasc Pharmacol. 2011 Nov;9(6):677-81. doi: 10.2174/157016111797484125. Curr Vasc Pharmacol. 2011. PMID: 21692740 Review.
-
High-mobility group box-1 and its role in angiogenesis.J Leukoc Biol. 2014 Apr;95(4):563-74. doi: 10.1189/jlb.0713412. Epub 2014 Jan 22. J Leukoc Biol. 2014. PMID: 24453275 Review.
Cited by
-
Serum high mobility group box-1 levels associated with cardiovascular events after lower extremity revascularization: a prospective study of a diabetic population.Cardiovasc Diabetol. 2022 Oct 16;21(1):214. doi: 10.1186/s12933-022-01650-1. Cardiovasc Diabetol. 2022. PMID: 36244983 Free PMC article.
-
High-mobility group box 1 fragment suppresses adverse post-infarction remodeling by recruiting PDGFRα-positive bone marrow cells.PLoS One. 2020 Apr 10;15(4):e0230392. doi: 10.1371/journal.pone.0230392. eCollection 2020. PLoS One. 2020. PMID: 32275672 Free PMC article.
-
Mechanisms of Non-Alcoholic Fatty Liver Disease in the Metabolic Syndrome. A Narrative Review.Antioxidants (Basel). 2021 Feb 10;10(2):270. doi: 10.3390/antiox10020270. Antioxidants (Basel). 2021. PMID: 33578702 Free PMC article. Review.
-
High-mobility group box 1 protein is implicated in advanced glycation end products-induced vascular endothelial growth factor A production in the rat retinal ganglion cell line RGC-5.Mol Vis. 2012;18:838-50. Epub 2012 Apr 5. Mol Vis. 2012. PMID: 22511847 Free PMC article.
-
Factors Influencing the Risk of Major Amputation in Patients with Diabetic Foot Ulcers Treated by Autologous Cell Therapy.J Diabetes Res. 2022 Apr 11;2022:3954740. doi: 10.1155/2022/3954740. eCollection 2022. J Diabetes Res. 2022. PMID: 35450383 Free PMC article.
References
-
- Martin A, Komada MR, Sane DC: Abnormal angiogenesis in diabetes mellitus. Med Res Rev 2003; 23: 117–145 - PubMed
-
- Waltenberger J: Impaired collateral vessel development in diabetes: potential cellular mechanisms and therapeutic implications. Cardiovasc Res 2001; 49: 554–560 - PubMed
-
- Scaffidi P, Misteli T, Bianchi ME: Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002; 418: 191–195 - PubMed

