PTEN inhibition improves muscle regeneration in mice fed a high-fat diet
- PMID: 20200318
- PMCID: PMC2874691
- DOI: 10.2337/db09-1155
PTEN inhibition improves muscle regeneration in mice fed a high-fat diet
Abstract
Objective: Mechanisms impairing wound healing in diabetes are poorly understood. To identify mechanisms, we induced insulin resistance by chronically feeding mice a high-fat diet (HFD). We also examined the regulation of phosphatidylinositol 3,4,5-trisphosphate (PIP(3)) during muscle regeneration because augmented IGF-1 signaling can improve muscle regeneration.
Research design and methods: Muscle regeneration was induced by cardiotoxin injury, and we evaluated satellite cell activation and muscle maturation in HFD-fed mice. We also measured PIP(3) and the enzymes regulating its level, IRS-1-associated phosphatidylinositol 3-kinase (PI3K) and PTEN. Using primary cultures of muscle, we examined how fatty acids affect PTEN expression and how PTEN knockout influences muscle growth. Mice with muscle-specific PTEN knockout were used to examine how the HFD changes muscle regeneration.
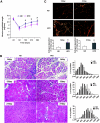
Results: The HFD raised circulating fatty acids and impaired the growth of regenerating myofibers while delaying myofiber maturation and increasing collagen deposition. These changes were independent of impaired proliferation of muscle progenitor or satellite cells but were principally related to increased expression of PTEN, which reduced PIP(3) in muscle. In cultured muscle cells, palmitate directly stimulated PTEN expression and reduced cell growth. Knocking out PTEN restored cell growth. In mice, muscle-specific PTEN knockout improved the defects in muscle repair induced by HFD.
Conclusions: Insulin resistance impairs muscle regeneration by preventing myofiber maturation. The mechanism involves fatty acid-stimulated PTEN expression, which lowers muscle PIP(3). If similar pathways occur in diabetic patients, therapeutic strategies directed at improving the repair of damaged muscle could include suppression of PTEN activity.
Figures

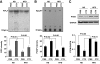



References
-
- Hirsch T, Spielmann M, Velander P, Zuhaili B, Bleiziffer O, Fossum M, Steinstraesser L, Yao F, Eriksson E: Insulin-like growth factor-1 gene therapy and cell transplantation in diabetic wounds. J Gene Med 2008; 10: 1247–1252 - PubMed
-
- Vignaud A, Ramond F, Hourde C, Keller A, Butler-Browne G, Ferry A: Diabetes provides an unfavorable environment for muscle mass and function after muscle injury in mice. Pathobiology 2007; 74: 291–300 - PubMed
-
- Shortreed K, Johnston A, Hawke TJ: Satellite cells and muscle repair. In Skeletal Muscle Damage and Repair 1st ed Tiidus PM: Ed. Champaign, IL, Human Kinetics, 2008, p. 77–88
-
- Grefte S, Kuijpers-Jagtman AM, Torensma R, Von den Hoff JW: Skeletal muscle development and regeneration. Stem Cells Dev 2007; 16: 857–868 - PubMed
-
- Charge SB, Rudnicki MA: Cellular and molecular regulation of muscle regeneration. Physiol Rev 2004; 84: 209–238 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

