Human embryonic stem cells: a source of mast cells for the study of allergic and inflammatory diseases
- PMID: 20200352
- PMCID: PMC2865868
- DOI: 10.1182/blood-2009-08-237206
Human embryonic stem cells: a source of mast cells for the study of allergic and inflammatory diseases
Abstract
Human mast cells are tissue resident cells with a principal role in allergic disorders. Cross-linking of the high-affinity receptor for immunoglobulin E (FcepsilonRI) results in release of inflammatory mediators initiating the clinical symptoms of allergy and anaphylaxis. Much of our knowledge regarding the mechanisms of mast cell activation comes from studies of mouse bone marrow-derived mast cells. However, clear differences have been identified between human and mouse mast cells. Studies of human mast cells are hampered by the limited sources available for their isolation, the resistance of these cells to genetic manipulation, and differences between cultures established from different persons. To address this limitation, we developed a simple coculture-free method for obtaining mast cells from human embryonic stem cells (hES). These hES-derived mast cells respond to antigen by releasing mast cell mediators. Moreover, the cells can be generated in numbers sufficient for studies of the pathways involved in their effector functions. Genetically modified mast cells, such as GFP-expressing cells, can be obtained by introduction and selection for modification in hES cells before differentiation. This direct coculture-free differentiation of hES cells represents a new and unique model to analyze the function and development of human mast cells.
Figures
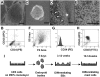
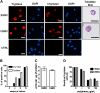
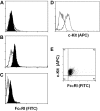
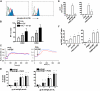
Similar articles
-
Differentiation of mast cells from embryonic stem cells.Curr Protoc Immunol. 2012 Apr;Chapter 22:Unit 22F.10.1-16. doi: 10.1002/0471142735.im22f10s97. Curr Protoc Immunol. 2012. PMID: 22470136
-
Development, migration, and survival of mast cells.Immunol Res. 2006;34(2):97-115. doi: 10.1385/IR:34:2:97. Immunol Res. 2006. PMID: 16760571 Free PMC article. Review.
-
Gene expression profiles for Fc epsilon RI, cytokines and chemokines upon Fc epsilon RI activation in human cultured mast cells derived from peripheral blood.Cytokine. 2001 Nov 21;16(4):143-52. doi: 10.1006/cyto.2001.0958. Cytokine. 2001. PMID: 11792124
-
Transgenic mice expressing the human high-affinity immunoglobulin (Ig) E receptor alpha chain respond to human IgE in mast cell degranulation and in allergic reactions.J Exp Med. 1996 Jan 1;183(1):49-56. doi: 10.1084/jem.183.1.49. J Exp Med. 1996. PMID: 8551243 Free PMC article.
-
Current Strategies to Inhibit High Affinity FcεRI-Mediated Signaling for the Treatment of Allergic Disease.Front Immunol. 2019 Feb 7;10:175. doi: 10.3389/fimmu.2019.00175. eCollection 2019. Front Immunol. 2019. PMID: 30792720 Free PMC article. Review.
Cited by
-
Rapid Mast Cell Generation from Gata2 Reporter Pluripotent Stem Cells.Stem Cell Reports. 2018 Oct 9;11(4):1009-1020. doi: 10.1016/j.stemcr.2018.08.007. Epub 2018 Sep 6. Stem Cell Reports. 2018. PMID: 30197119 Free PMC article.
-
The role of macrophage migration inhibitory factor in mast cell-stimulated fibroblast proliferation and collagen production.PLoS One. 2015 Mar 31;10(3):e0122482. doi: 10.1371/journal.pone.0122482. eCollection 2015. PLoS One. 2015. PMID: 25826375 Free PMC article.
-
Mast Cell-Biomaterial Interactions and Tissue Repair.Tissue Eng Part B Rev. 2021 Dec;27(6):590-603. doi: 10.1089/ten.TEB.2020.0275. Epub 2021 Jan 21. Tissue Eng Part B Rev. 2021. PMID: 33164714 Free PMC article. Review.
-
Advances in cellular technology in the hematology field: What have we learned so far?World J Stem Cells. 2015 Jan 26;7(1):106-15. doi: 10.4252/wjsc.v7.i1.106. World J Stem Cells. 2015. PMID: 25621110 Free PMC article. Review.
-
Generating hematopoietic cells from human pluripotent stem cells: approaches, progress and challenges.Cell Regen. 2023 Sep 1;12(1):31. doi: 10.1186/s13619-023-00175-6. Cell Regen. 2023. PMID: 37656237 Free PMC article. Review.
References
-
- Kovarova M, Rivera J. A molecular understanding of mast cell activation and the promise of anti-allergic therapeutics. Curr Med Chem. 2004;11(15):2083–2091. - PubMed
-
- Kraft S, Kinet JP. New developments in FcepsilonRI regulation, function and inhibition. Nat Rev Immunol. 2007;7(5):365–378. - PubMed
-
- Nguyen M, Solle M, Audoly LP, et al. Receptors and signaling mechanisms required for prostaglandin E2-mediated regulation of mast cell degranulation and IL-6 production. J Immunol. 2002;169(8):4586–4593. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

