Vitamin C transporter Slc23a1 links renal reabsorption, vitamin C tissue accumulation, and perinatal survival in mice
- PMID: 20200446
- PMCID: PMC2847422
- DOI: 10.1172/JCI39191
Vitamin C transporter Slc23a1 links renal reabsorption, vitamin C tissue accumulation, and perinatal survival in mice
Abstract
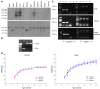
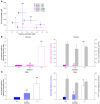
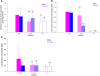
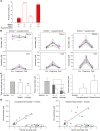
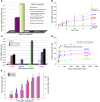
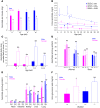
Levels of the necessary nutrient vitamin C (ascorbate) are tightly regulated by intestinal absorption, tissue accumulation, and renal reabsorption and excretion. Ascorbate levels are controlled in part by regulation of transport through at least 2 sodium-dependent transporters: Slc23a1 and Slc23a2 (also known as Svct1 and Svct2, respectively). Previous work indicates that Slc23a2 is essential for viability in mice, but the roles of Slc23a1 for viability and in adult physiology have not been determined. To investigate the contributions of Slc23a1 to plasma and tissue ascorbate concentrations in vivo, we generated Slc23a1-/- mice. Compared with wild-type mice, Slc23a1-/- mice increased ascorbate fractional excretion up to 18-fold. Hepatic portal ascorbate accumulation was nearly abolished, whereas intestinal absorption was marginally affected. Both heterozygous and knockout pups born to Slc23a1-/- dams exhibited approximately 45% perinatal mortality, and this was associated with lower plasma ascorbate concentrations in dams and pups. Perinatal mortality of Slc23a1-/- pups born to Slc23a1-/- dams was prevented by ascorbate supplementation during pregnancy. Taken together, these data indicate that ascorbate provided by the dam influenced perinatal survival. Although Slc23a1-/- mice lost as much as 70% of their ascorbate body stores in urine daily, we observed an unanticipated compensatory increase in ascorbate synthesis. These findings indicate a key role for Slc23a1 in renal ascorbate absorption and perinatal survival and reveal regulation of vitamin C biosynthesis in mice.
Figures
Similar articles
-
Gender and sodium-ascorbate transporter isoforms determine ascorbate concentrations in mice.J Nutr. 2004 Sep;134(9):2216-21. doi: 10.1093/jn/134.9.2216. J Nutr. 2004. PMID: 15333707
-
Regulation of vitamin C transport.Annu Rev Nutr. 2005;25:105-25. doi: 10.1146/annurev.nutr.25.050304.092647. Annu Rev Nutr. 2005. PMID: 16011461 Review.
-
Ascorbic-acid transporter Slc23a1 is essential for vitamin C transport into the brain and for perinatal survival.Nat Med. 2002 May;8(5):514-7. doi: 10.1038/0502-514. Nat Med. 2002. PMID: 11984597
-
Genetic variation in the sodium-dependent vitamin C transporters, SLC23A1, and SLC23A2 and risk for preterm delivery.Am J Epidemiol. 2006 Feb 1;163(3):245-54. doi: 10.1093/aje/kwj035. Epub 2005 Dec 15. Am J Epidemiol. 2006. PMID: 16357110
-
Sodium-dependent ascorbic acid transporter family SLC23.Pflugers Arch. 2004 Feb;447(5):677-82. doi: 10.1007/s00424-003-1104-1. Epub 2003 Jul 4. Pflugers Arch. 2004. PMID: 12845532 Review.
Cited by
-
Human genetic variation influences vitamin C homeostasis by altering vitamin C transport and antioxidant enzyme function.Annu Rev Nutr. 2013;33:45-70. doi: 10.1146/annurev-nutr-071812-161246. Epub 2013 Apr 29. Annu Rev Nutr. 2013. PMID: 23642198 Free PMC article. Review.
-
Lost-in-Translation of Metabolic Effects of Inorganic Nitrate in Type 2 Diabetes: Is Ascorbic Acid the Answer?Int J Mol Sci. 2021 Apr 29;22(9):4735. doi: 10.3390/ijms22094735. Int J Mol Sci. 2021. PMID: 33947005 Free PMC article. Review.
-
Nuclear receptor-mediated alleviation of alcoholic fatty liver by polyphenols contained in alcoholic beverages.PLoS One. 2014 Feb 3;9(2):e87142. doi: 10.1371/journal.pone.0087142. eCollection 2014. PLoS One. 2014. PMID: 24498295 Free PMC article.
-
Increased expression of SVCT2 in a new mouse model raises ascorbic acid in tissues and protects against paraquat-induced oxidative damage in lung.PLoS One. 2012;7(4):e35623. doi: 10.1371/journal.pone.0035623. Epub 2012 Apr 30. PLoS One. 2012. PMID: 22558179 Free PMC article.
-
Vitamin C transporter SVCT1 serves a physiological role as a urate importer: functional analyses and in vivo investigations.Pflugers Arch. 2023 Apr;475(4):489-504. doi: 10.1007/s00424-023-02792-1. Epub 2023 Feb 7. Pflugers Arch. 2023. PMID: 36749388 Free PMC article.
References
-
- Nishikimi M, Yagi K. Biochemistry and molecular biology of ascorbic acid biosynthesis. Subcell Biochem. 1996;25:17–39. - PubMed
-
- [No authors listed]. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 39-1995. A 72-year-old man with exertional dyspnea, fatigue, and extensive ecchymoses and purpuric lesions. N Engl J Med. 1995;333(25):1695–1702. doi: 10.1056/NEJM199512213332508. - DOI - PubMed
-
- Food and Nutrition Board (U.S.R.C.)Recommended Dietary Allowances . Washington, DC: National Academy Press; 1989.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

