Impact of lipid raft integrity on 5-HT3 receptor function and its modulation by antidepressants
- PMID: 20200506
- PMCID: PMC3055465
- DOI: 10.1038/npp.2010.20
Impact of lipid raft integrity on 5-HT3 receptor function and its modulation by antidepressants
Abstract
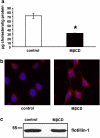
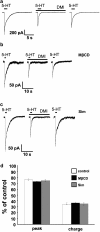
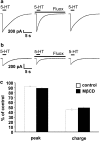
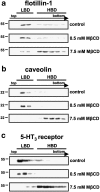
Because of the biochemical colocalization of the 5-HT(3) receptor and antidepressants within raft-like domains and their antagonistic effects at this ligand-gated ion channel, we investigated the impact of lipid raft integrity for 5-HT(3) receptor function and its modulation by antidepressants. Treatment with methyl-beta-cyclodextrine (MbetaCD) markedly reduced membrane cholesterol levels and caused a more diffuse membrane distribution of the lipid raft marker protein flotillin-1 indicating lipid raft impairment. Both amplitude and charge of serotonin evoked cation currents were diminished following cholesterol depletion by either MbetaCD or simvastatin (Sim), whereas the functional antagonistic properties of the antidepressants desipramine (DMI) and fluoxetine (Fluox) at the 5-HT(3) receptor were retained. Although both the 5-HT(3) receptor and flotillin-1 were predominantly found in raft-like domains in western blots following sucrose density gradient centrifugation, immunocytochemistry revealed only a coincidental degree of colocalization of these two proteins. These findings and the persistence of the antagonistic effects of DMI and Fluox against 5-HT(3) receptors after lipid raft impairment indicate that their modulatory effects are likely mediated through non-raft 5-HT(3) receptors, which are not sufficiently detected by means of sucrose density gradient centrifugation. In conclusion, lipid raft integrity appears to be important for 5-HT(3) receptor function in general, whereas it is not a prerequisite for the antagonistic properties of antidepressants such as DMI and Fluox at this ligand-gated ion channel.
Figures

Similar articles
-
Modulation of ligand-gated ion channels as a novel pharmacological principle.Pharmacopsychiatry. 2011 May;44 Suppl 1:S27-34. doi: 10.1055/s-0031-1271704. Epub 2011 May 4. Pharmacopsychiatry. 2011. PMID: 21544743
-
Lipid raft integrity affects GABAA receptor, but not NMDA receptor modulation by psychopharmacological compounds.Int J Neuropsychopharmacol. 2013 Jul;16(6):1361-71. doi: 10.1017/S146114571200140X. Epub 2012 Dec 10. Int J Neuropsychopharmacol. 2013. PMID: 23217923
-
Antidepressants and antipsychotic drugs colocalize with 5-HT3 receptors in raft-like domains.J Neurosci. 2005 Nov 2;25(44):10198-206. doi: 10.1523/JNEUROSCI.2460-05.2005. J Neurosci. 2005. PMID: 16267227 Free PMC article.
-
Antidepressants are functional antagonists at the serotonin type 3 (5-HT3) receptor.Mol Psychiatry. 2003 Nov;8(12):994-1007. doi: 10.1038/sj.mp.4001314. Mol Psychiatry. 2003. PMID: 14647397
-
Direct and indirect cholesterol effects on membrane proteins with special focus on potassium channels.Biochim Biophys Acta Mol Cell Biol Lipids. 2020 Aug;1865(8):158706. doi: 10.1016/j.bbalip.2020.158706. Epub 2020 Apr 1. Biochim Biophys Acta Mol Cell Biol Lipids. 2020. PMID: 32247032 Review.
Cited by
-
Presynaptic control of glycine transporter 2 (GlyT2) by physical and functional association with plasma membrane Ca2+-ATPase (PMCA) and Na+-Ca2+ exchanger (NCX).J Biol Chem. 2014 Dec 5;289(49):34308-24. doi: 10.1074/jbc.M114.586966. Epub 2014 Oct 14. J Biol Chem. 2014. PMID: 25315779 Free PMC article.
-
Allosteric modulation of the 5-HT(3) receptor.Curr Opin Pharmacol. 2011 Feb;11(1):75-80. doi: 10.1016/j.coph.2011.01.010. Epub 2011 Feb 20. Curr Opin Pharmacol. 2011. PMID: 21342788 Free PMC article. Review.
-
The role of intracellular linkers in gating and desensitization of human pentameric ligand-gated ion channels.J Neurosci. 2014 May 21;34(21):7238-52. doi: 10.1523/JNEUROSCI.5105-13.2014. J Neurosci. 2014. PMID: 24849357 Free PMC article.
-
Cryo-EM structures of prokaryotic ligand-gated ion channel GLIC provide insights into gating in a lipid environment.Nat Commun. 2024 Apr 5;15(1):2967. doi: 10.1038/s41467-024-47370-w. Nat Commun. 2024. PMID: 38580666 Free PMC article.
-
Tapping into 5-HT3 Receptors to Modify Metabolic and Immune Responses.Int J Mol Sci. 2021 Nov 2;22(21):11910. doi: 10.3390/ijms222111910. Int J Mol Sci. 2021. PMID: 34769340 Free PMC article. Review.
References
-
- Allen JA, Halverson-Tamboli RA, Rasenick MM. Lipid raft microdomains and neurotransmitter signalling. Nat Rev Neurosci. 2007;8:128–140. - PubMed
-
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. - PubMed
-
- Barrantes FJ. Cholesterol effects on nicotinic acetylcholine receptor. J Neurochem. 2007;103 (Suppl 1:72–80. - PubMed
-
- Baumann P, Gaillard JM, Perey M, Justafre JC, Le P. Relationships between brain concentrations of desipramine and paradoxical sleep inhibition in the rat. J Neural Transm. 1983;56:105–116. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

