IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses
- PMID: 20200520
- PMCID: PMC2861732
- DOI: 10.1038/nature08901
IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses
Abstract
CD4(+) T helper 2 (T(H)2) cells secrete interleukin (IL)4, IL5 and IL13, and are required for immunity to gastrointestinal helminth infections. However, T(H)2 cells also promote chronic inflammation associated with asthma and allergic disorders. The non-haematopoietic-cell-derived cytokines thymic stromal lymphopoietin, IL33 and IL25 (also known as IL17E) have been implicated in inducing T(H)2 cell-dependent inflammation at mucosal sites, but how these cytokines influence innate immune responses remains poorly defined. Here we show that IL25, a member of the IL17 cytokine family, promotes the accumulation of a lineage-negative (Lin(-)) multipotent progenitor (MPP) cell population in the gut-associated lymphoid tissue that promotes T(H)2 cytokine responses. The IL25-elicited cell population, termed MPP(type2) cells, was defined by the expression of Sca-1 (also known as Ly6a) and intermediate expression of c-Kit (c-Kit(int)), and exhibited multipotent capacity, giving rise to cells of monocyte/macrophage and granulocyte lineages both in vitro and in vivo. Progeny of MPP(type2) cells were competent antigen presenting cells, and adoptive transfer of MPP(type2) cells could promote T(H)2 cytokine responses and confer protective immunity to helminth infection in normally susceptible Il25(-/-) mice. The ability of IL25 to induce the emergence of an MPP(type2) cell population identifies a link between the IL17 cytokine family and extramedullary haematopoiesis, and suggests a previously unrecognized innate immune pathway that promotes T(H)2 cytokine responses at mucosal sites.
Conflict of interest statement
Unless otherwise stated, the authors declare no competing financial interests.
Figures

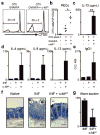
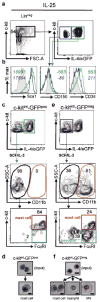
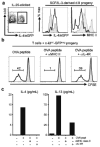
Comment in
-
Immunology: Close encounters of the second type.Nature. 2010 Apr 29;464(7293):1285-6. doi: 10.1038/4641285a. Nature. 2010. PMID: 20428154 No abstract available.
References
-
- Umetsu DT, McIntire JJ, Akbari O, Macaubas C, DeKruyff RH. Asthma: an epidemic of dysregulated immunity. Nat Immunol. 2002;3:715–720. - PubMed
-
- Soumelis V, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. - PubMed
-
- Schmitz J, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. - PubMed
-
- Fort MM, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI074878/AI/NIAID NIH HHS/United States
- T32AI007532-08/AI/NIAID NIH HHS/United States
- R01 AI095466/AI/NIAID NIH HHS/United States
- AI074878/AI/NIAID NIH HHS/United States
- T32 HL007775/HL/NHLBI NIH HHS/United States
- P30 DK50306/DK/NIDDK NIH HHS/United States
- R01 AI061570/AI/NIAID NIH HHS/United States
- F31GM082187/GM/NIGMS NIH HHS/United States
- AI61570/AI/NIAID NIH HHS/United States
- P30 DK050306/DK/NIDDK NIH HHS/United States
- T32 AI007532/AI/NIAID NIH HHS/United States
- AI083480/AI/NIAID NIH HHS/United States
- R01 AI102942/AI/NIAID NIH HHS/United States
- R21 AI083480/AI/NIAID NIH HHS/United States
- F31 GM082187/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

