Cutaneous vaccination using microneedles coated with hepatitis C DNA vaccine
- PMID: 20200562
- PMCID: PMC2914565
- DOI: 10.1038/gt.2010.22
Cutaneous vaccination using microneedles coated with hepatitis C DNA vaccine
Abstract
The skin is potentially an excellent organ for vaccine delivery because of accessibility and the presence of immune cells. However, no simple and inexpensive cutaneous vaccination method is available. Micron-scale needles coated with DNA were tested as a simple, inexpensive device for skin delivery. Vaccination with a plasmid encoding hepatitis C virus nonstructural 3/4A protein using microneedles effectively primed specific cytotoxic T lymphocytes (CTLs). Importantly, the minimally invasive microneedles were as efficient in priming CTLs as more complicated or invasive delivery techniques, such as gene gun and hypodermic needles. Thus, microneedles may offer a promising technology for DNA vaccination.
Figures
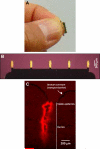
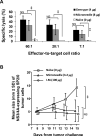
References
-
- Garmory HS, Perkins SD, Phillpotts RJ, Titball RW. DNA vaccines for biodefence. Adv Drug Deliver Rev. 2005;57:1343–1361. - PubMed
-
- Donnelly J, Berry K, Ulmer JB. Technical and regulatory hurdles for DNA vaccines. Int J Parasitol. 2003;33:457–467. - PubMed
-
- Nicolas JF, Guy B. Intradermal, epidermal and transcutaneous vaccination: from immunology to clinical practice. Expert Rev Vaccines. 2008;7:1201–1214. - PubMed
-
- Prausnitz MR, Mikszta JA, Cormier M, Andrianov AK. Microneedle-based vaccines. In: Compans RW, Orenstein WA, editors. Current Topics in Microbiology & Immunology: Vaccines for Pandemic Influenza. Springer-Verlag; Berlin/Heidelberg, Germany: 2009.
-
- Matriano JA, Cormier M, Johnson J, Young WA, Buttery M, Nyam K, et al. Macroflux microprojection array patch technology: a new and efficient approach for intracutaneous immunization. Pharm Res. 2002;19:63–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

