Progress and prospects: nuclear import of nonviral vectors
- PMID: 20200566
- PMCID: PMC4084667
- DOI: 10.1038/gt.2010.31
Progress and prospects: nuclear import of nonviral vectors
Abstract
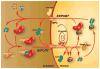
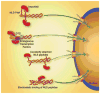
The nuclear envelope represents a key barrier to successful nonviral transfection and gene therapy both in vitro and in vivo. Although the main purpose of the nuclear envelope is to partition the cell to maintain cytoplasmic components in the cytoplasm and nuclear components, most notably genomic DNA, in the nucleus, this function poses a problem for transfections in which exogenous DNA is delivered into the cytoplasm. After delivery to the cytoplasm, nucleic acids rapidly become complexed with cellular proteins that mediate interactions with the cellular machinery for trafficking. Thus, it is these proteins that, in essence, control the nuclear import of DNA, and we must also understand their activities in cells. In this review, we will discuss the principles of nuclear import of proteins and DNA-protein complexes, as well as the various approaches that investigators have used to improve nuclear targeting of plasmids. These approaches include complexation of plasmids with peptides, native and engineered proteins, ligands and polymers, as well as the inclusion of transcription factor-binding sites for general and cell-specific delivery.
Keywords: nonviral gene transfermid R:plasmidmid R:nuclear pore complexmid R:importinmid R:nuclear localization signalmid R:karyopherin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Intracellular trafficking of plasmids for gene therapy: mechanisms of cytoplasmic movement and nuclear import.Curr Gene Ther. 2006 Dec;6(6):671-681. doi: 10.2174/156652306779010688. Curr Gene Ther. 2006. PMID: 17168698 Free PMC article. Review.
-
Emerging significance of plasmid DNA nuclear import in gene therapy.Adv Drug Deliv Rev. 2003 Jun 16;55(6):749-60. doi: 10.1016/s0169-409x(03)00050-4. Adv Drug Deliv Rev. 2003. PMID: 12788538 Review.
-
Incorporation of a Nuclear Localization Signal in pH Responsive LAH4-L1 Peptide Enhances Transfection and Nuclear Uptake of Plasmid DNA.Mol Pharm. 2016 Sep 6;13(9):3141-52. doi: 10.1021/acs.molpharmaceut.6b00338. Epub 2016 Aug 9. Mol Pharm. 2016. PMID: 27458925
-
Cytoplasmic transport and nuclear import of plasmid DNA.Biosci Rep. 2017 Nov 29;37(6):BSR20160616. doi: 10.1042/BSR20160616. Print 2017 Dec 22. Biosci Rep. 2017. PMID: 29054961 Free PMC article. Review.
-
Ultrasound microbubbles combined with the NFκB binding motif increase transfection efficiency by enhancing the cytoplasmic and nuclear import of plasmid DNA.Mol Med Rep. 2013 Nov;8(5):1439-45. doi: 10.3892/mmr.2013.1672. Epub 2013 Sep 9. Mol Med Rep. 2013. PMID: 24026477
Cited by
-
Nanomedicine for gene therapy.Drug Deliv Transl Res. 2013 Oct;3(5):437-45. doi: 10.1007/s13346-012-0120-0. Drug Deliv Transl Res. 2013. PMID: 25788352
-
Noninvasive cell-tracking methods.Nat Rev Clin Oncol. 2011 Sep 27;8(11):677-88. doi: 10.1038/nrclinonc.2011.141. Nat Rev Clin Oncol. 2011. PMID: 21946842 Review.
-
The role of surface chemistry-induced cell characteristics on nonviral gene delivery to mouse fibroblasts.J Biol Eng. 2012 Sep 11;6(1):17. doi: 10.1186/1754-1611-6-17. J Biol Eng. 2012. PMID: 22967455 Free PMC article.
-
Polyplex exposure inhibits cell cycle, increases inflammatory response, and can cause protein expression without cell division.Mol Pharm. 2013 Apr 1;10(4):1306-17. doi: 10.1021/mp300470d. Epub 2013 Mar 21. Mol Pharm. 2013. PMID: 23458572 Free PMC article.
-
Lipopolyplex for Therapeutic Gene Delivery and Its Application for the Treatment of Parkinson's Disease.Front Aging Neurosci. 2016 Apr 5;8:68. doi: 10.3389/fnagi.2016.00068. eCollection 2016. Front Aging Neurosci. 2016. PMID: 27092073 Free PMC article. Review.
References
-
- Miller AM, Dean DA. Tissue-specific and transcription factor-mediated nuclear entry of DNA. Adv Drug Deliv Rev. 2009;61:603–613. - PubMed
-
- Glover DJ, Leyton DL, Moseley GW, Jans DA. The efficiency of nuclear plasmid DNA delivery is a critical determinant of transgene expression at the single cell level. J Gene Med. 2010;12:77–85. - PubMed
-
- McLane LM, Corbett AH. Nuclear localization signals and human disease. IUBMB Life. 2009;61:697–706. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

