Time Controlled Protein Release from Layer-by-Layer Assembled Multilayer Functionalized Agarose Hydrogels
- PMID: 20200599
- PMCID: PMC2830720
- DOI: 10.1002/adfm.200901172
Time Controlled Protein Release from Layer-by-Layer Assembled Multilayer Functionalized Agarose Hydrogels
Abstract
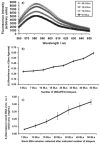
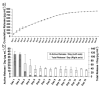
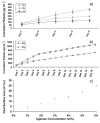
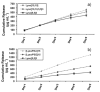
Axons of the adult central nervous system exhibit an extremely limited ability to regenerate after spinal cord injury. Experimentally generated patterns of axon growth are typically disorganized and randomly oriented. Support of linear axonal growth into spinal cord lesion sites has been demonstrated using arrays of uniaxial channels, templated with agarose hydrogel, and containing genetically engineered cells that secrete brain-derived neurotrophic factor (BDNF). However, immobilizing neurotrophic factors secreting cells within a scaffold is relatively cumbersome, and alternative strategies are needed to provide sustained release of BDNF from templated agarose scaffolds. Existing methods of loading the drug or protein into hydrogels cannot provide sustained release from templated agarose hydrogels. Alternatively, here it is shown that pH-responsive H-bonded poly(ethylene glycol)(PEG)/poly(acrylic acid)(PAA)/protein hybrid layer-by-layer (LbL) thin films, when prepared over agarose, provided sustained release of protein under physiological conditions for more than four weeks. Lysozyme, a protein similar in size and isoelectric point to BDNF, is released from the multilayers on the agarose and is biologically active during the earlier time points, with decreasing activity at later time points. This is the first demonstration of month-long sustained protein release from an agarose hydrogel, whereby the drug/protein is loaded separately from the agarose hydrogel fabrication process.
Figures




References
-
- Stokols S, Sakamoto J, Breckon C, Holt T, Weiss J, Tuszynski MH. Tissue Eng. 2006;12:2777. - PubMed
-
- Tuszynski MH, Gabriel K, Gage FH, Suhr S, Meyer S, Rosetti A. Exp Neurol. 1996;137:157. - PubMed
-
- Blesch A, Tuszynski MH. J Comp Neurol. 2003;467:403. - PubMed
-
- Bradbury EJ, Khemani S, King VR, Priestley JV, McMahon SB. Eur J Neurosci. 1999;11:3873. - PubMed
-
- Menei P, Montero-Menei C, Whittemore SR, Bunge RP, Bunge MB. Eur J Neurosci. 1998;10:607. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
