Roles of retrotransposons in benign and malignant hematologic disease
- PMID: 20200603
- PMCID: PMC2830787
Roles of retrotransposons in benign and malignant hematologic disease
Abstract
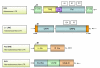
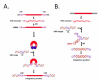
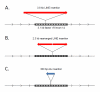
Nearly half of our genomes are repetitive sequences derived from retrotransposons. These repeats have accumulated by a 'copy-and-paste' mechanism whereby: (i.) a genomic template sequence is transcribed to RNA, (ii.) the RNA is reverse-transcribed, and (iii.) the DNA copy is inserted at a new location in the host genome. As we remain susceptible to new retrotransposition events, many of these insertions are highly polymorphic. Transposons are of interest since insertions into both coding and non-coding gene regions have been associated with a wide variety of functional sequelae and because transposable elements can be involved in genomic rearrangements in transformed cells. In this review, we highlight how expression of retrotransposons, de novo and polymorphic transposon insertions, and genomic rearrangements that these repeats potentiate contribute to both benign and neoplastic hematopoietic diseases.
Figures

Similar articles
-
The identification of retro-DNAs in primate genomes as DNA transposons mobilizing via retrotransposition.F1000Res. 2024 May 29;12:255. doi: 10.12688/f1000research.130043.3. eCollection 2023. F1000Res. 2024. PMID: 38915770 Free PMC article.
-
Ginger DNA transposons in eukaryotes and their evolutionary relationships with long terminal repeat retrotransposons.Mob DNA. 2010 Jan 25;1(1):3. doi: 10.1186/1759-8753-1-3. Mob DNA. 2010. PMID: 20226081 Free PMC article.
-
Transcriptional landscape of repetitive elements in normal and cancer human cells.BMC Genomics. 2014 Jul 11;15:583. doi: 10.1186/1471-2164-15-583. BMC Genomics. 2014. PMID: 25012247 Free PMC article.
-
Human transposon tectonics.Cell. 2012 May 11;149(4):740-52. doi: 10.1016/j.cell.2012.04.019. Cell. 2012. PMID: 22579280 Free PMC article. Review.
-
Regulation of retrotransposition in Arabidopsis.Biochem Soc Trans. 2021 Nov 1;49(5):2241-2251. doi: 10.1042/BST20210337. Biochem Soc Trans. 2021. PMID: 34495315 Review.
Cited by
-
Evidence for the persistence of an active endogenous retrovirus (ERVE) in humans.Genetica. 2014 Oct;142(5):451-60. doi: 10.1007/s10709-014-9789-y. Epub 2014 Sep 6. Genetica. 2014. PMID: 25192754
-
The cancer-associated CTCFL/BORIS protein targets multiple classes of genomic repeats, with a distinct binding and functional preference for humanoid-specific SVA transposable elements.Epigenetics Chromatin. 2016 Aug 31;9(1):35. doi: 10.1186/s13072-016-0084-2. eCollection 2016. Epigenetics Chromatin. 2016. PMID: 27588042 Free PMC article.
-
MIR retrotransposons link the epigenome and the transcriptome of coding genes in acute myeloid leukemia.Nat Commun. 2022 Oct 31;13(1):6524. doi: 10.1038/s41467-022-34211-x. Nat Commun. 2022. PMID: 36316347 Free PMC article.
-
Inter-Strain Differences in LINE-1 DNA Methylation in the Mouse Hematopoietic System in Response to Exposure to Ionizing Radiation.Int J Mol Sci. 2017 Jul 4;18(7):1430. doi: 10.3390/ijms18071430. Int J Mol Sci. 2017. PMID: 28677663 Free PMC article.
-
Fuel source shift or cost reduction: Context-dependent adaptation strategies in closely related Neodon fuscus and Lasiopodomys brandtii against hypoxia.Zool Res. 2022 Jul 18;43(4):497-513. doi: 10.24272/j.issn.2095-8137.2022.011. Zool Res. 2022. PMID: 35585802 Free PMC article.
References
-
- Kazazian HH, Jr., Wong C, Youssoufian H, Scott AF, Phillips DG, Antonarakis SE. Haemophilia A resulting from de novo insertion of L1 sequences represents a novel mechanism for mutation in man. Nature. 1988;332:164–166. - PubMed
-
- Belancio VP, Hedges DJ, Deininger P. Mammalian non-LTR retrotransposons: for better or worse, in sickness and in health. Genome Res. 2008;18:343–358. - PubMed
-
- Chen JM, Chuzhanova N, Stenson PD, Ferec C, Cooper DN. Meta-analysis of gross insertions causing human genetic disease: novel mutational mechanisms and the role of replication slippage. Hum Mutat. 2005;25:207–221. - PubMed
-
- Jones JM, Gellert M. The taming of a transposon: V(D)J recombination and the immune system. Immunol Rev. 2004;200:233–248. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
