Tumor-derived syndecan-1 mediates distal cross-talk with bone that enhances osteoclastogenesis
- PMID: 20200931
- PMCID: PMC3148092
- DOI: 10.1002/jbmr.16
Tumor-derived syndecan-1 mediates distal cross-talk with bone that enhances osteoclastogenesis
Abstract
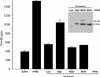
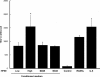
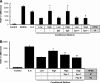
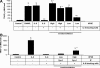
Tumor-stimulated bone resorption fuels tumor growth and marks a dramatic decline in the health and prognosis of breast cancer patients. Identifying mechanisms that mediate cross-talk between tumor and bone remains a key challenge. We previously demonstrated that breast cancer cells expressing high levels of heparanase exhibit enhanced shedding of the syndecan-1 proteoglycan. Moreover, when these heparanase-high cells are implanted in the mammary fat pad, they elevate bone resorption. In this study, conditioned medium from breast cancer cells expressing high levels of heparanase was shown to significantly stimulate human osteoclastogenesis in vitro (p < .05). The osteoclastogenic activity in the medium of heparanase-high cells was traced to the presence of syndecan-1, intact heparan sulfate chains, and heat-labile factor(s), including the chemokine interleukin 8 (IL-8). The enhanced osteoclastogenesis promoted by the heparanase-high cells results in a dramatic increase in bone resorption in vitro. In addition, the long bones of animals bearing heparanase-high tumors in the mammary fat pad had significantly higher numbers of osteoclasts compared with animals bearing tumors expressing low levels of heparanase (p < .05). Together these data suggest that syndecan-1 shed by tumor cells exerts biologic effects distal to the primary tumor and that it participates in driving osteoclastogenesis and the resulting bone destruction.
(c) 2010 American Society for Bone and Mineral Research.
Figures

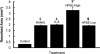
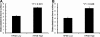
References
-
- Kakonen SM, Mundy GR. Mechanisms of osteolytic bone metastases in breast carcinoma. Cancer. 2003;97:834–839. - PubMed
-
- Bendre MS, Gaddy-Kurten D, Mon-Foote T, et al. Expression of interleukin 8 and not parathyroid hormone-related protein by human breast cancer cells correlates with bone metastasis in vivo. Cancer Res. 2002;62:5571–5579. - PubMed
-
- Kang Y, Siegel PM, Shu W, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

