Sterile inflammatory responses mediated by the NLRP3 inflammasome
- PMID: 20201012
- PMCID: PMC3601805
- DOI: 10.1002/eji.200940207
Sterile inflammatory responses mediated by the NLRP3 inflammasome
Abstract
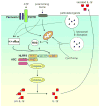
Through pattern recognition receptors the innate immune system detects disruption of the normal function of the organism and initiates responses directed at correcting these derangements. Cellular damage from microbial or non-microbial insults causes the activation of nucleotide-binding domain leucine-rich repeat containing receptors in multiprotein complexes called inflammasomes. Here we discuss the role of the NLRP3 inflammasome in the recognition of cellular damage and the initiation of sterile inflammatory responses.
Conflict of interest statement
Figures
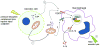
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

