Autocitrullination of human peptidyl arginine deiminase type 4 regulates protein citrullination during cell activation
- PMID: 20201080
- PMCID: PMC2951335
- DOI: 10.1002/art.27439
Autocitrullination of human peptidyl arginine deiminase type 4 regulates protein citrullination during cell activation
Abstract
Objective: To address mechanisms that control the activity of human peptidyl arginine deiminase type 4 (PAD-4).
Methods: PAD-4 autocitrullination was determined by anti-modified citrulline immunoblotting, using purified recombinant and endogenous PAD-4 from activated human primary neutrophils and cell lines expressing PAD-4. The citrullination sites in PAD-4 were determined by mass spectrometry. Mechanisms of autocitrullination-induced inactivation and the functional consequences of autocitrullination in PAD-4 polymorphic variants were addressed using purified components and cell lines expressing PAD-4 wild-type, PAD-4 mutant, and PAD-4 polymorphic variants relevant to rheumatoid arthritis (RA).
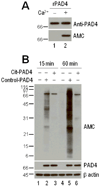
Results: PAD-4 is autocitrullinated in vitro and during activation of primary cells and cell lines expressing PAD-4. Interestingly, this modification inactivated the function of the enzyme. The efficiency of inactivation differed among genetically defined PAD-4 variants relevant to RA. PAD-4 was citrullinated at 10 sites, which are clustered into 3 distinct regions, including a cluster of arginines around the active site cleft where Arg-372 and -374 were identified as the potential autocitrullination targets that inactivate the enzyme. Autocitrullination also modified the structure of PAD-4, abrogating its recognition by multiple rabbit antibodies, but augmenting its recognition by human anti-PAD-4 autoantibodies.
Conclusion: Our findings suggest that autocitrullination regulates the production of citrullinated proteins during cell activation, and that this is affected by structural polymorphisms in PAD-4. Autocitrullination also influences PAD-4 structure and immune response.
Figures
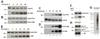
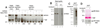



References
-
- Gelato KA, Fischle W. Role of histone modifications in defining chromatin structure and function. Biol Chem. 2008;389(4):353–363. - PubMed
-
- Tarcsa E, Marekov LN, Mei G, Melino G, Lee SC, Steinert PM. Protein unfolding by peptidylarginine deiminase. Substrate specificity and structural relationships of the natural substrates trichohyalin and filaggrin. J Biol Chem. 1996;271(48):30709–30716. - PubMed
-
- Doyle HA, Mamula MJ. Posttranslational modifications of self-antigens. Ann N Y Acad Sci. 2005;1050:1–9. - PubMed
-
- Rogers GE. Occurrence of citrulline in proteins. Nature. 1962;194:1149–1151. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

