Nitric oxide, oxygen, and superoxide formation and consumption in macrophages and colonic epithelial cells
- PMID: 20201482
- PMCID: PMC2879621
- DOI: 10.1021/tx900415k
Nitric oxide, oxygen, and superoxide formation and consumption in macrophages and colonic epithelial cells
Abstract
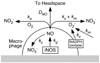
Knowledge of the rates at which macrophages and epithelial cells synthesize NO is critical for predicting the concentrations of NO and other reactive nitrogen species in colonic crypts during inflammation, and elucidating the linkage between inflammatory bowel disease, NO, and cancer. Macrophage-like RAW264.7 cells, primary bone marrow-derived macrophages (BMDM), and HCT116 colonic epithelial cells were subjected to simulated inflammatory conditions, and rates of formation and consumption were determined for NO, O(2), and O(2)(-). Production rates of NO were determined in either of two ways: continuous monitoring of NO concentrations in a closed chamber with corrections for autoxidation, or NO(2)(-) accumulation measurements in an open system with corrections for diffusional losses of NO. The results obtained using the two methods were in excellent agreement. Rates of NO synthesis (2.3 +/- 0.6 pmol s(-1) 10(6) cells(-1)), NO consumption (1.3 +/- 0.3 s(-1)), and O(2) consumption (59 +/- 17 pmol s(-1) 10(6) cells(-1) when NO is negligible) for activated BMDM were indistinguishable from those of activated RAW264.7 cells. NO production rates calculated from NO(2)(-) accumulation data for HCT116 cells infected with Helicobacter cinaedi (3.9 +/- 0.1 pmol s(-1) 10(6) cells(-1)) were somewhat greater than those of RAW264.7 macrophages infected under similar conditions (2.6 +/- 0.1 pmol s(-1) 10(6) cells(-1)). Thus, RAW264.7 cells have NO kinetics nearly identical to those of primary macrophages, and stimulated epithelial cells are capable of synthesizing NO at rates comparable to those of macrophages. Using these cellular kinetic parameters, simulations of NO diffusion and reaction in a colonic crypt during inflammation predict maximum NO concentrations of about 0.2 microM at the base of a crypt.
Figures






References
-
- Tamir S, Tannenbaum SR. The role of nitric oxide in the carcinogenic process. Biochim Biophys Acta. 1996;1288:31–36. - PubMed
-
- Levin B. Ulcerative colitis and colon cancer: biology and surveillance. J Cell Biochem Suppl. 1993;16G:47–50. - PubMed
-
- Farrell RJ, Peppercorn MA. Ulcerative colitis. Lancet. 2002;359:331–340. - PubMed
-
- Singer I, Kawka DW, Scott S, Weidner JR, Mumford RA, Riehl TE, Stenson WF. Expression of inducible nitric oxide synthase and nitrotyrosine in colonic epithelium in inflammatory bowel disease. Gastroenterology. 1996;111:871–885. - PubMed
-
- Kimura H, Hokari R, Miura S, Shigetmatsu T, Hirokawa M, Akiba Y, Kurose I, Higuchi H, Fujimori H, Tsuzuki Y, Serizawa H, Ishii H. Increased expression of an inducible isoform of nitric oxide synthase and the formation of peroxynitrite in colonic mucosa of patients with active ulcerative colitis. Gut. 1998;42:180–187. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

