Coordination modes of multidentate ligands in fac-[Re(CO)(3)(polyaminocarboxylate)] analogues of (99m)Tc radiopharmaceuticals. dependence on aqueous solution reaction conditions
- PMID: 20201565
- PMCID: PMC2861859
- DOI: 10.1021/ic9017568
Coordination modes of multidentate ligands in fac-[Re(CO)(3)(polyaminocarboxylate)] analogues of (99m)Tc radiopharmaceuticals. dependence on aqueous solution reaction conditions
Abstract
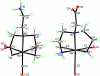
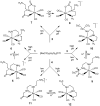
We study Re analogues of (99m)Tc renal agents to interpret previous results at the (99m)Tc tracer level. The relative propensities of amine donors versus carboxylate oxygen donors of four L = polyaminocarboxylate ligands to coordinate in fac-[Re(I)(CO)(3)L](n) complexes were assessed by examining the reaction of fac-[Re(I)(CO)(3)(H(2)O)(3)](+) under conditions differing in acidity and temperature. All four L [N,N-bis-(2-aminoethyl)glycine (DTGH), N,N-ethylenediaminediacetic acid, diethylenetriamine-N-malonic acid, and diethylenetriamine-N-acetic acid] can coordinate as tridentate ligands while creating a dangling chain terminated in a carboxyl group. Dangling carboxyl groups facilitate renal clearance in fac-[(99m)Tc(I)(CO)(3)L](n) agents. Under neutral conditions, the four ligands each gave two fac-[Re(I)(CO)(3)L](n) products with HPLC traces correlating well with known traces of the fac-[(99m)Tc(I)(CO)(3)L](n) mixtures. Such mixtures are common in renal agents because the needed dangling carboxyl group can compete for a coordination site. However, the HPLC separations needed to assess the biodistribution of a single tracer are impractical in a clinical setting. One goal in investigating this Re chemistry is to identify conditions for avoiding this problem of mixtures in preparations of fac-[(99m)Tc(I)(CO)(3)L](n) renal tracers. After separation and isolation of the fac-[Re(I)(CO)(3)L](n) products, NMR analysis of all products and single crystal X-ray crystallographic analysis of both DTGH products, as well as one product each from the other L, allowed us to establish coordination mode unambiguously. The product favored in acidic conditions has a dangling amine chain and more bound oxygen. The product favored in basic conditions has a dangling carboxyl chain and more bound nitrogen. At the elevated temperatures used for simulating tracer preparation, equilibration was facile (ca. 1 h or less), allowing selective formation of one product by utilizing acidic or basic conditions. The results of this fundamental study offer protocols and guidance useful for the design and preparation of fac-[(99m)Tc(I)(CO)(3)L](n) agents consisting of a single tracer.
Figures





References
-
- Richards P. In: Technetium in Chemistry and Nuclear Medicine 3. Nicolini M, Bandoli G, Mazzi U, editors. Cortina International; Verona, Italy: 1990. pp. 5–9.
-
- Bandoli G, Dolmella A, Porchia M, Refosco F, Tisato F. Coord. Chem. Rev. 2001;214:43–90.
-
- Lipowska M, Cini R, Tamasi G, Xu X, Taylor AT, Marzilli LG. Inorg. Chem. 2004;43:7774–7783. - PubMed
-
- He H, Lipowska M, Xu X, Taylor AT, Carlone M, Marzilli LG. Inorg. Chem. 2005;44:5437–5446. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

