The biology of the sodium iodide symporter and its potential for targeted gene delivery
- PMID: 20201784
- PMCID: PMC3916908
- DOI: 10.2174/156800910791054194
The biology of the sodium iodide symporter and its potential for targeted gene delivery
Abstract
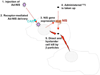
The sodium iodide symporter (NIS) is responsible for thyroidal, salivary, gastric, intestinal and mammary iodide uptake. It was first cloned from the rat in 1996 and shortly thereafter from human and mouse tissue. In the intervening years, we have learned a great deal about the biology of NIS. Detailed knowledge of its genomic structure, transcriptional and post-transcriptional regulation and pharmacological modulation has underpinned the selection of NIS as an exciting approach for targeted gene delivery. A number of in vitro and in vivo studies have demonstrated the potential of using NIS gene therapy as a means of delivering highly conformal radiation doses selectively to tumours. This strategy is particularly attractive because it can be used with both diagnostic (99mTc, 125I, 124I)) and therapeutic (131I, 186Re, 188Re, 211At) radioisotopes and it lends itself to incorporation with standard treatment modalities, such as radiotherapy or chemoradiotherapy. In this article, we review the biology of NIS and discuss its development for gene therapy.
Figures



References
-
- Dohan O, De la Vieja A, Paroder V, Riedel C, Artani M, Reed M, Ginter CS, Carrasco N. The sodium/iodide symporter (NIS): characterization, regulation, and medical significance. En-docr. Rev. 2003;24:48–77. - PubMed
-
- Haq MS, Harmer C. Thyroid cancer: an overview. Nucl. Med. Commun. 2004;25:861–867. - PubMed
-
- Baumann E. Uber den Jodgehalt der Schilddrusen von Menchen und tieren. Hoppe-Seyler ‘s Zeitschrift fur Physiologische Chemie. 1986;22:1–17.
-
- Spitzweg C, Harrington KJ, Pinke LA, Vile RG, Morris JC. Clinical review 132: The sodium iodide symporter and its potential role in cancer therapy. J. Clin. Endocrinol. Metab. 2001;86:3327–3335. - PubMed
-
- Eskandari S, Loo DD, Dai G, Levy O, Wright EM, Carrasco N. Thyroid Na+/I- symporter Mechanism, stoichiometry, and specificity. J. Biol. Chem. 1997;272:27230–27238. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

