The Vpu protein: new concepts in virus release and CD4 down-modulation
- PMID: 20201792
- PMCID: PMC4290667
- DOI: 10.2174/157016210791111124
The Vpu protein: new concepts in virus release and CD4 down-modulation
Abstract
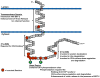
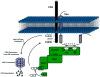
Human immunodeficiency virus type 1 (HIV-1) and several simian immunodeficiency viruses (SIV) encode for a transmembrane protein known as Vpu (viral protein U). While one of the smallest of the HIV-1 proteins, it has two important functions within virus-infected cells. The first of these functions is the down-regulation of the CD4 receptor to prevent its interaction with the HIV-1 envelope glycoprotein. Vpu interacts with the CD4 receptor in the rough endoplasmic reticulum (RER), resulting in its re-translocation across the RER and subsequent degradation via the proteasomal pathway. The second major function of the Vpu protein is to facilitate release of virus from infected cells. Previous studies have shown that virus release is cell type specific, suggesting that certain cells may express a restriction factor that inhibits virus release in the absence of Vpu. Recently, bone marrow stromal antigen 2 (BST-2/HM1.24/CD317/tetherin) has been identified as this factor. This review will focus on new findings within the last four years on the role of Vpu in CD4 down-regulation and the restriction of virus release from cells. We will relate these findings to virus pathogenesis and propose questions regarding BST-2 as a restriction factor.
Figures

References
-
- Besnard-Guerin C, Belaidouni N, Lassot I, Segeral E, Jobart A, Marchal C, Benarous R. Journal of Biological Chemistry. 2004;279:788–795. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

