Cardiac myocyte force development during differentiation and maturation
- PMID: 20201894
- PMCID: PMC2920416
- DOI: 10.1111/j.1749-6632.2009.05091.x
Cardiac myocyte force development during differentiation and maturation
Abstract
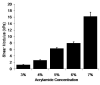
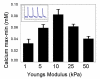
The maturation of cardiac myocytes during the immediate prenatal period coincides with changes in the mechanical properties of the extracellular matrix. We investigated the effects of extracellular stiffness on cardiomyocyte maturation in neonatal rat ventricular myocytes grown on collagen-coated gels. Cells on 10-kPa substrates developed aligned sarcomeres, while cells on stiffer substrates had unaligned sarcomeres and stress fibers. Cells generated greater mechanical force on gels with stiffness similar to that of the native myocardium than on stiffer or softer substrates. To investigate the differentiation of myocyte progenitors, we used clonal expansion of engineered human embryonic stem cells. Puromycin-selected cardiomyocytes exhibited a gene expression profile similar to that of adult human cardiomyocytes and generated force and action potentials consistent with normal fetal cardiomyocytes. These results suggest that extracellular stiffness significantly affects maturation and differentiation of immature ventricular myocytes.
Figures




Similar articles
-
Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes.Biophys J. 2008 Oct;95(7):3479-87. doi: 10.1529/biophysj.107.124545. Epub 2008 Jun 27. Biophys J. 2008. PMID: 18586852 Free PMC article.
-
Non-cardiomyocytes influence the electrophysiological maturation of human embryonic stem cell-derived cardiomyocytes during differentiation.Stem Cells Dev. 2010 Jun;19(6):783-95. doi: 10.1089/scd.2009.0349. Stem Cells Dev. 2010. PMID: 20001453 Free PMC article.
-
Maturation of human embryonic stem cell-derived cardiomyocytes (hESC-CMs) in 3D collagen matrix: Effects of niche cell supplementation and mechanical stimulation.Acta Biomater. 2017 Feb;49:204-217. doi: 10.1016/j.actbio.2016.11.058. Epub 2016 Nov 24. Acta Biomater. 2017. PMID: 27890729
-
Mechanobiology of cardiomyocyte development.J Biomech. 2010 Jan 5;43(1):93-8. doi: 10.1016/j.jbiomech.2009.09.014. Epub 2009 Oct 12. J Biomech. 2010. PMID: 19819458 Free PMC article. Review.
-
Effect of substrate mechanics on cardiomyocyte maturation and growth.Tissue Eng Part B Rev. 2015 Feb;21(1):157-65. doi: 10.1089/ten.TEB.2014.0383. Epub 2014 Nov 12. Tissue Eng Part B Rev. 2015. PMID: 25148904 Free PMC article. Review.
Cited by
-
Prospective isolation of human embryonic stem cell-derived cardiovascular progenitors that integrate into human fetal heart tissue.Proc Natl Acad Sci U S A. 2013 Feb 26;110(9):3405-10. doi: 10.1073/pnas.1220832110. Epub 2013 Feb 7. Proc Natl Acad Sci U S A. 2013. PMID: 23391730 Free PMC article.
-
In vitro aged, hiPSC-origin engineered heart tissue models with age-dependent functional deterioration to study myocardial infarction.Acta Biomater. 2019 Aug;94:372-391. doi: 10.1016/j.actbio.2019.05.064. Epub 2019 May 27. Acta Biomater. 2019. PMID: 31146032 Free PMC article.
-
Rigid microenvironments promote cardiac differentiation of mouse and human embryonic stem cells.Sci Technol Adv Mater. 2013 Aug 1;14(2):025003. doi: 10.1088/1468-6996/14/2/025003. Sci Technol Adv Mater. 2013. PMID: 24311969 Free PMC article.
-
Substrate stiffness increases twitch power of neonatal cardiomyocytes in correlation with changes in myofibril structure and intracellular calcium.Biophys J. 2011 Nov 16;101(10):2455-64. doi: 10.1016/j.bpj.2011.09.057. Epub 2011 Nov 15. Biophys J. 2011. PMID: 22098744 Free PMC article.
-
A biomaterial approach to cell reprogramming and differentiation.J Mater Chem B. 2017 Apr 7;5(13):2375-2379. doi: 10.1039/C6TB03130G. Epub 2017 Feb 20. J Mater Chem B. 2017. PMID: 28966790 Free PMC article.
References
-
- Thompson R-P, et al. Collagen synthesis in the developing chick heart. Tex Rep Biol Med. 1979;39:305–19. - PubMed
-
- Pelham RJ, JR., Wang YL. Cell locomotion and focal adhesions are regulated by the mechanical properties of the substrate. Biol Bull. 1998;194:348–9. discussion 349-50. - PubMed
-
- Brown XQ, Ookawa K, Wong JY. Evaluation of polydimethylsiloxane scaffolds with physiologically-relevant elastic moduli: interplay of substrate mechanics and surface chemistry effects on vascular smooth muscle cell response. Biomaterials. 2005;26:3123–9. - PubMed
-
- Genes NG, et al. Effect of substrate mechanics on chondrocyte adhesion to modified alginate surfaces. Arch Biochem Biophys. 2004;422:161–7. - PubMed
-
- Peyton SR, et al. The use of poly(ethylene glycol) hydrogels to investigate the impact of ECM chemistry and mechanics on smooth muscle cells. Biomaterials. 2006;27:4881–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

