Ethanol impairs activation of retinoic acid receptors in cerebellar granule cells in a rodent model of fetal alcohol spectrum disorders
- PMID: 20201933
- PMCID: PMC4502960
- DOI: 10.1111/j.1530-0277.2010.01166.x
Ethanol impairs activation of retinoic acid receptors in cerebellar granule cells in a rodent model of fetal alcohol spectrum disorders
Abstract
Background: Ethanol is the main addictive and neurotoxic constituent of alcohol. Ethanol exposure during embryonic development causes dysfunction of the central nervous system (CNS) and leads to fetal alcohol spectrum disorders. The cerebellum is one of the CNS regions that are particularly vulnerable to ethanol toxic effects. Retinoic acid (RA) is a physiologically active metabolite of vitamin A that is locally synthesized in the cerebellum. Studies have shown that RA is required for neuronal development, but it remains unknown if ethanol impairs RA signaling and thus induces neuronal malformations. In this study, we tested the hypothesis that ethanol impairs the expression and activation of RA receptors in cerebellum and in cerebellar granule cells.
Methods: The cerebellum of ethanol unexposed and exposed pups was used to study the expression of retinoic acid receptors (RARs or RXRs) by immunohistochemistry and by Western blot analysis. We also studied the effect of ethanol on expression of RA receptors in the cerebellar granule cells. Activation of RA receptors (DNA-binding activities) in response to high-dose ethanol was determined by electrophoretic mobility shift and supershift assays.
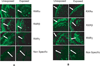
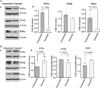
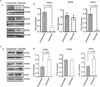
Results: Findings from these studies demonstrated that ethanol exposure reduced the expression of RARalpha/gamma while it increased the expression of RXRalpha/gamma in the cerebellum and in cerebellar granule neurons. Immuno-histological studies further strengthened the expression pattern of RA receptors in response to ethanol. The DNA-binding activity of RARs was reduced, while DNA-binding activity of RXRs was increased in response to ethanol exposure.
Conclusion: For the first time, our studies have demonstrated that high-dose ethanol affects the expression and activation of RA receptors, which could impair the signaling events and induce harmful effects on the survival and differentiation of cerebellar granule cells. Taken together, these findings could provide insight into the treatment options for brain defects caused by excessive ethanol exposure, such as in Fetal Alcohol Spectrum Disorders.
Figures




Similar articles
-
Mechanisms of ethanol-induced death of cerebellar granule cells.Cerebellum. 2012 Mar;11(1):145-54. doi: 10.1007/s12311-010-0219-0. Cerebellum. 2012. PMID: 20927663 Free PMC article. Review.
-
Mechanisms of Ethanol-Induced Cerebellar Ataxia: Underpinnings of Neuronal Death in the Cerebellum.Int J Environ Res Public Health. 2021 Aug 18;18(16):8678. doi: 10.3390/ijerph18168678. Int J Environ Res Public Health. 2021. PMID: 34444449 Free PMC article. Review.
-
Resveratrol restores Nrf2 level and prevents ethanol-induced toxic effects in the cerebellum of a rodent model of fetal alcohol spectrum disorders.Mol Pharmacol. 2011 Sep;80(3):446-57. doi: 10.1124/mol.111.071126. Epub 2011 Jun 22. Mol Pharmacol. 2011. PMID: 21697273 Free PMC article.
-
Ethanol impairs Rho GTPase signaling and differentiation of cerebellar granule neurons in a rodent model of fetal alcohol syndrome.Cell Mol Life Sci. 2006 Dec;63(23):2859-70. doi: 10.1007/s00018-006-6333-y. Cell Mol Life Sci. 2006. PMID: 17109064 Free PMC article.
-
Acid-sensitive channel inhibition prevents fetal alcohol spectrum disorders cerebellar Purkinje cell loss.Am J Physiol Regul Integr Comp Physiol. 2008 Aug;295(2):R596-603. doi: 10.1152/ajpregu.90321.2008. Epub 2008 May 28. Am J Physiol Regul Integr Comp Physiol. 2008. PMID: 18509098 Free PMC article.
Cited by
-
Resveratrol prevents impairment in activation of retinoic acid receptors and MAP kinases in the embryos of a rodent model of diabetic embryopathy.Reprod Sci. 2012 Sep;19(9):949-61. doi: 10.1177/1933719112438972. Epub 2012 Apr 24. Reprod Sci. 2012. PMID: 22534330 Free PMC article.
-
Comparison of molecular marker expression in early zebrafish brain development following chronic ethanol or morpholino treatment.Exp Brain Res. 2017 Aug;235(8):2413-2423. doi: 10.1007/s00221-017-4977-5. Epub 2017 May 10. Exp Brain Res. 2017. PMID: 28493069 Free PMC article.
-
Mechanisms of ethanol-induced death of cerebellar granule cells.Cerebellum. 2012 Mar;11(1):145-54. doi: 10.1007/s12311-010-0219-0. Cerebellum. 2012. PMID: 20927663 Free PMC article. Review.
-
Alcohol exposure decreases CREB binding protein expression and histone acetylation in the developing cerebellum.PLoS One. 2011;6(5):e19351. doi: 10.1371/journal.pone.0019351. Epub 2011 May 31. PLoS One. 2011. PMID: 21655322 Free PMC article.
-
Mechanisms of Ethanol-Induced Cerebellar Ataxia: Underpinnings of Neuronal Death in the Cerebellum.Int J Environ Res Public Health. 2021 Aug 18;18(16):8678. doi: 10.3390/ijerph18168678. Int J Environ Res Public Health. 2021. PMID: 34444449 Free PMC article. Review.
References
-
- Altman J. Postnatal development of the cerebellar cortex in the rat. 3. Maturation of the components of the granular layer. J Comp Neurol. 1972a;145:465–513. - PubMed
-
- Altman J. Postnatal development of the cerebellar cortex in the rat. II. Phases in the maturation of Purkinje cells and of the molecular layer. J Comp Neurol. 1972b;145:399–463. - PubMed
-
- Altucci L, Leibowitz MD, Ogilvie KM, de Lera AR, Gronemeyer H. RAR and RXR modulation in cancer and metabolic disease. Nat Rev Drug Discov. 2007;6:793–810. - PubMed
-
- Bauer-Moffett C, Altman J. The effect of ethanol chronically administered to preweanling rats on cerebellar development: a morphological study. Brain Res. 1977;119:249–268. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

