Salmonella pathogenicity island 1 differentially modulates bacterial entry to dendritic and non-phagocytic cells
- PMID: 20201987
- PMCID: PMC2878471
- DOI: 10.1111/j.1365-2567.2009.03233.x
Salmonella pathogenicity island 1 differentially modulates bacterial entry to dendritic and non-phagocytic cells
Abstract
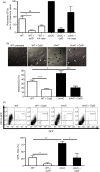
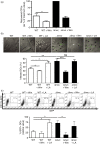
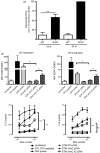
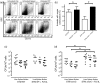
Salmonella enterica serovar Typhimurium can enter non-phagocytic cells, such as intestinal epithelial cells, by virtue of a Type Three Secretion System (TTSS) encoded in the Salmonella Pathogenicity Island 1 (SPI-1), which translocates bacterial effector molecules into the host cell. Salmonella can also be taken up by dendritic cells (DCs). Although the role of SPI-1 in non-phagocytic cell invasion is well established, its contribution to invasion of phagocytic cells has not been evaluated. Here, we have tested the invasive capacity of a S. Typhimurium strain lacking a key component of its TTSS-1 (DeltaInvC) leading to defective translocation of SPI-1-encoded effectors. Whereas this mutant Salmonella strain was impaired for invasion of non-phagocytic cells, it was taken up by DCs at a significantly higher rate than wild-type Salmonella. Similar to wild-type Salmonella, the DeltaInvC mutant strain retained the capacity to avoid antigen presentation to T cells. However, mice infected with the DeltaInvC mutant strain showed higher survival rate and reduced organ colonization. Our data suggest that, besides promoting phagocytosis by non-phagocytic cells, SPI-1 modulates the number of bacteria that enters DCs. The SPI-1 could be considered not only as an inducer of epithelial cell invasion but as a controller of DC entry.
Figures


References
-
- Ohl ME, Miller SI. Salmonella: a model for bacterial pathogenesis. Annu Rev Med. 2001;52:259–74. - PubMed
-
- Clark MA, Jepson MA, Simmons NL, Hirst BH. Preferential interaction of Salmonella typhimurium with mouse Peyer’s patch M cells. Res Microbiol. 1994;145:543–52. - PubMed
-
- Jepson MA, Clark MA. The role of M cells in Salmonella infection. Microbes Infect. 2001;3:1183–90. - PubMed
-
- Hopkins SA, Niedergang F, Corthesy-Theulaz IE, Kraehenbuhl JP. A recombinant Salmonella typhimurium vaccine strain is taken up and survives within murine Peyer’s patch dendritic cells. Cell Microbiol. 2000;2:59–68. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

