Histone deacetylase inhibitor vorinostat suppresses the growth of uterine sarcomas in vitro and in vivo
- PMID: 20202195
- PMCID: PMC2843655
- DOI: 10.1186/1476-4598-9-49
Histone deacetylase inhibitor vorinostat suppresses the growth of uterine sarcomas in vitro and in vivo
Abstract
Background: Uterine sarcomas are very rare malignancies with no approved chemotherapy protocols. Histone deacetylase (HDAC) inhibitors belong to the most promising groups of compounds for molecular targeting therapy. Here, we described the antitumor effects of suberoylanilide hydroxamic acid (SAHA; vorinostat) on MES-SA uterine sarcoma cells in vitro and in vivo. We investigated effects of vorinostat on growth and colony forming ability by using uterine sarcoma MES-SA cells. We analyzed the influence of vorinostat on expression of different HDACs, p21(WAF1) and activation of apoptosis. Finally, we examined the antitumor effects of vorinostat on uterine sarcoma in vivo.
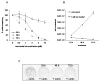
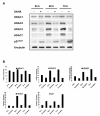
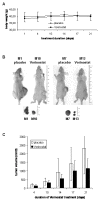
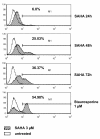
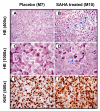
Results: Vorinostat efficiently suppressed MES-SA cell growth at a low dosage (3 microM) already after 24 hours treatment. Decrease of cell survival was even more pronounced after prolonged treatment and reached 9% and 2% after 48 and 72 hours of treatment, respectively. Colony forming capability of MES-SA cells treated with 3 microM vorinostat for 24 and 48 hours was significantly diminished and blocked after 72 hours. HDACs class I (HDAC2 and 3) as well as class II (HDAC7) were preferentially affected by this treatment. Vorinostat significantly increased p21(WAF1) expression and apoptosis. Nude mice injected with 5 x 106 MES-SA cells were treated for 21 days with vorinostat (50 mg/kg/day) and, in comparison to placebo group, a tumor growth reduction of more than 50% was observed. Results obtained by light- and electron-microscopy suggested pronounced activation of apoptosis in tumors isolated from vorinostat-treated mice.
Conclusions: Our data strongly indicate the high therapeutic potential of vorinostat in uterine sarcomas.
Figures

References
-
- Harker WG, MacKintosh FR, Sikic BI. Development and characterization of a human sarcoma cell line, MES-SA, sensitive to multiple drugs. Cancer Res. 1983;43:4943–4950. - PubMed
-
- Marks PA, Richon VM, Miller T, Kelly WK. Histone deacetylase inhibitors. Adv Cancer Res. 2004;91:137–168. full_text. - PubMed

