Morphologic characterization of osteosarcoma growth on the chick chorioallantoic membrane
- PMID: 20202196
- PMCID: PMC2838906
- DOI: 10.1186/1756-0500-3-58
Morphologic characterization of osteosarcoma growth on the chick chorioallantoic membrane
Abstract
Background: The chick chorio-allantoic membrane (CAM) assay is a commonly used method for studying angiogenic or anti-angiogenic activities in vivo. The ease of access allows direct monitoring of tumour growth by biomicroscopy and the possibility to screen many samples in an inexpensive way. The CAM model provides a powerful tool to study effects of molecules, which interfere with physiological angiogenesis, or experimental tumours derived from cancer cell lines. We therefore screened eight osteosarcoma cell lines for their ability to form vascularized tumours on the CAM.
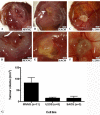
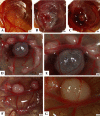
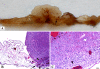
Findings: We implanted 3-5 million cells of human osteosarcoma lines (HOS, MG63, MNNG-HOS, OST, SAOS, SJSA1, U2OS, ZK58) on the CAM at day 10 of embryonic development. Tumour growth was monitored by in vivo biomicroscopy at different time points and tumours were fixed in paraformaldehyde seven days after cell grafting. The tissue was observed, photographed and selected cases were further analyzed using standard histology.From the eight cell lines the MNNG-HOS, U2OS and SAOS were able to form solid tumours when grafted on the CAM. The MNNG-HOS tumours showed the most reliable and consistent growth and were able to penetrate the chorionic epithelium, grow in the CAM stroma and induce a strong angiogenic response.
Conclusions: Our results show that the CAM assay is a useful tool for studying osteosarcoma growth. The model provides an excellent alternative to current rodent models and could serve as a preclinical screening assay for anticancer molecules. It might increase the speed and efficacy of the development of new drugs for the treatment of osteosarcoma.
Figures
Similar articles
-
Optimization of the chicken chorioallantoic membrane assay as reliable in vivo model for the analysis of osteosarcoma.PLoS One. 2019 Apr 15;14(4):e0215312. doi: 10.1371/journal.pone.0215312. eCollection 2019. PLoS One. 2019. PMID: 30986223 Free PMC article.
-
Experimental tumor growth of canine osteosarcoma cell line on chick embryo chorioallantoic membrane (in vivo studies).Acta Vet Scand. 2017 May 12;59(1):30. doi: 10.1186/s13028-017-0298-8. Acta Vet Scand. 2017. PMID: 28499392 Free PMC article.
-
Inhibitory effect of a mixture containing ascorbic acid, lysine, proline and green tea extract on critical parameters in angiogenesis.Oncol Rep. 2005 Oct;14(4):807-15. Oncol Rep. 2005. PMID: 16142336
-
Chick embryo chorioallantoic membrane as a useful tool to study angiogenesis.Int Rev Cell Mol Biol. 2008;270:181-224. doi: 10.1016/S1937-6448(08)01405-6. Int Rev Cell Mol Biol. 2008. PMID: 19081537 Review.
-
The chick chorioallantoic membrane (CAM) as a versatile patient-derived xenograft (PDX) platform for precision medicine and preclinical research.Am J Cancer Res. 2018 Aug 1;8(8):1642-1660. eCollection 2018. Am J Cancer Res. 2018. PMID: 30210932 Free PMC article. Review.
Cited by
-
The Chicken Embryo Chorioallantoic Membrane as an In Vivo Model for Photodynamic Therapy.Methods Mol Biol. 2022;2451:107-125. doi: 10.1007/978-1-0716-2099-1_9. Methods Mol Biol. 2022. PMID: 35505014 Review.
-
E2F1 regulates cellular growth by mTORC1 signaling.PLoS One. 2011 Jan 24;6(1):e16163. doi: 10.1371/journal.pone.0016163. PLoS One. 2011. PMID: 21283628 Free PMC article.
-
Effect of Laryngeal Squamous Cell Carcinoma Tissue Implantation on the Chick Embryo Chorioallantoic Membrane: Morphometric Measurements and Vascularity.Biomed Res Int. 2015;2015:629754. doi: 10.1155/2015/629754. Epub 2015 Oct 11. Biomed Res Int. 2015. PMID: 26539518 Free PMC article.
-
Characterization of liposarcoma cell lines for preclinical and biological studies.Sarcoma. 2012;2012:148614. doi: 10.1155/2012/148614. Epub 2012 Jul 14. Sarcoma. 2012. PMID: 22911243 Free PMC article.
-
The CAM Model-Q&A with Experts.Cancers (Basel). 2022 Dec 28;15(1):191. doi: 10.3390/cancers15010191. Cancers (Basel). 2022. PMID: 36612187 Free PMC article. Review.
References
-
- Winkler K, Beron G, Delling G, Heise U, Kabisch H, Purfurst C, Berger J, Ritter J, Jurgens H, Gerein V. Neoadjuvant chemotherapy of osteosarcoma: results of a randomized cooperative trial (COSS-82) with salvage chemotherapy based on histological tumor response. J Clin Oncol. 1988;6:329–337. - PubMed
LinkOut - more resources
Full Text Sources

