Dynamics of peptidergic secretory granule transport are regulated by neuronal stimulation
- PMID: 20202202
- PMCID: PMC2838897
- DOI: 10.1186/1471-2202-11-32
Dynamics of peptidergic secretory granule transport are regulated by neuronal stimulation
Abstract
Background: Peptidergic neurons store and secrete the contents of large dense core vesicles (LDCVs) from axon terminals and from dendrites. Secretion of peptides requires a highly regulated exocytotic mechanism, plus coordinated synthesis and transport of LDCVs to their sites of release. Although these trafficking events are critical to function, little is known regarding the dynamic behavior of LDCVs and the mechanisms by which their transport is regulated. Sensory neurons also package opiate receptors in peptide-containing LDCVs, which is thought to be important in pain sensation. Since peptide granules cannot be refilled locally after their contents are secreted, it is particularly important to understand how neurons support regulated release of peptides.
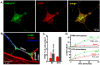
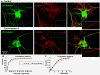
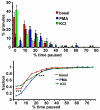
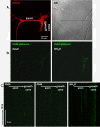
Results: A vector encoding soluble peptidylglycine alpha-hydroxylating monooxygenase fused to green fluorescent protein was constructed to address these questions in cultured primary peptidergic neurons of the trigeminal ganglion using time lapse confocal microscopy. The time course of release differs with secretagogue; the secretory response to depolarization with K+ is rapid and terminates within 15 minutes, while phorbol ester stimulation of secretion is maintained over a longer period. The data demonstrate fundamental differences between LDCV dynamics in axons and growth cones under basal conditions.
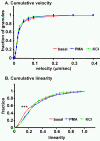
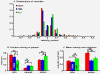
Conclusions: Under basal conditions, LDCVs move faster away from the soma than toward the soma, but fewer LDCVs travel anterograde than retrograde. Stimulation decreased average anterograde velocity and increases granule pausing. Data from antibody uptake, quantification of enzyme secretion and appearance of pHluorin fluorescence demonstrate distributed release of peptides all along the axon, not just at terminals.
Figures



Similar articles
-
Differences in the ways sympathetic neurons and endocrine cells process, store, and secrete exogenous neuropeptides and peptide-processing enzymes.J Neurosci. 1999 Oct 1;19(19):8300-11. doi: 10.1523/JNEUROSCI.19-19-08300.1999. J Neurosci. 1999. PMID: 10493731 Free PMC article.
-
Mechanisms of transport and exocytosis of dense-core granules containing tissue plasminogen activator in developing hippocampal neurons.J Neurosci. 2005 Mar 23;25(12):3095-106. doi: 10.1523/JNEUROSCI.4694-04.2005. J Neurosci. 2005. PMID: 15788766 Free PMC article.
-
Secretory vesicle trafficking in awake and anaesthetized mice: differential speeds in axons versus synapses.J Physiol. 2018 Aug;596(16):3759-3773. doi: 10.1113/JP276022. Epub 2018 Jul 1. J Physiol. 2018. PMID: 29873393 Free PMC article.
-
From endoplasmic reticulum to secretory granules: role of zinc in the secretory pathway of growth hormone.Endocr Dev. 2012;23:96-108. doi: 10.1159/000341763. Epub 2012 Nov 23. Endocr Dev. 2012. PMID: 23182824 Review.
-
Red, yellow, green go!--A novel tool for microscopic segregation of secretory vesicle pools according to their age.Biochem Soc Trans. 2003 Aug;31(Pt 4):851-6. doi: 10.1042/bst0310851. Biochem Soc Trans. 2003. PMID: 12887320 Review.
Cited by
-
Neuronal activity in the hub of extrasynaptic Schwann cell-axon interactions.Front Cell Neurosci. 2013 Nov 25;7:228. doi: 10.3389/fncel.2013.00228. eCollection 2013. Front Cell Neurosci. 2013. PMID: 24324401 Free PMC article.
-
Neuropeptides and small-molecule amine transmitters: cooperative signaling in the nervous system.Cell Mol Life Sci. 2022 Aug 23;79(9):492. doi: 10.1007/s00018-022-04451-7. Cell Mol Life Sci. 2022. PMID: 35997826 Free PMC article. Review.
-
Cognitive stimulation in the workplace, plasma proteins, and risk of dementia: three analyses of population cohort studies.BMJ. 2021 Aug 18;374:n1804. doi: 10.1136/bmj.n1804. BMJ. 2021. PMID: 34407988 Free PMC article.
-
Corticotropin-releasing factor-dopamine interactions in male and female macaque: Beyond the classic VTA.Synapse. 2024 Jan;78(1):e22284. doi: 10.1002/syn.22284. Epub 2023 Nov 23. Synapse. 2024. PMID: 37996987 Free PMC article.
-
Stac protein regulates release of neuropeptides.Proc Natl Acad Sci U S A. 2020 Nov 24;117(47):29914-29924. doi: 10.1073/pnas.2009224117. Epub 2020 Nov 9. Proc Natl Acad Sci U S A. 2020. PMID: 33168737 Free PMC article.
References
-
- Takamori S, Holt M, Stenius K, Lemke EA, Gronborg M, Riedel D, Urlaub H, Schenck S, Brugger B, Ringler P, Muller SA, Rammner B, Grater F, Hub JS, De Groot BL, Mieskes G, Moriyama Y, Klingauf J, Grubmuller H, Heuser J, Wieland F, Jahn R. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. - DOI - PubMed
-
- Mains RE, Eipper BA. In: Basic Neurochemistry, Molecular, Cellular and Medical Aspects. 7. Siegel GR, Albers W, Brady ST, Price DL, editor. New York: Elsevier; 2006. Peptides; pp. 317–332.
-
- Kaether C, Salm T, Glombik M, Almers W, Gerdes HH. Targeting of green fluorescent protein to neuroendocrine secretory granules: a new tool for real time studies of regulated protein secretion. Eur J Cell Biol. 1997;74:133–142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

