Self-association of Zn-insulin at neutral pH: investigation by concentration gradient--static and dynamic light scattering
- PMID: 20202736
- PMCID: PMC2867077
- DOI: 10.1016/j.bpc.2010.02.001
Self-association of Zn-insulin at neutral pH: investigation by concentration gradient--static and dynamic light scattering
Abstract
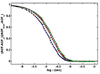
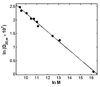
Equilibrium self-association of Zn-insulin at pH 7.0 was characterized over the range 0.3-5mg/mL by simultaneous measurement of static and dynamic light scattering. Analysis of static light scattering yielded a concentration-dependent weight-average molecular weight, and analysis of dynamic light scattering yielded a concentration-dependent intensity-average diffusion coefficient. The concentration dependence of both quantities may be accounted for to within experimental precision by a simple model, according to which the basic structural unit of Zn-insulin at concentrations exceeding 0.3mg/mL is a hexamer H. With increasing total protein concentration, hexameric protomers may self-associate in accordance with an isodesmic scheme in which a protomer may add to any prexisting oligomer H(n) to form H(n+1) with an invariant stepwise equilibrium association constant.
Figures





References
-
- Dunn MF. Zinc-ligand interactions modulate assembly and stability of the insulin hexamer - a review. Biometals. 2005;18:295–303. - PubMed
-
- Vallee BL, Gibson JG., 2nd The zinc content of normal human whole blood, plasma, leucocytes, and erythrocytes. J Biol Chem. 1948;176:445–457. - PubMed
-
- Milthorpe BK, Nichol LW, Jeffrey PD. The polymerization pattern of zinc(II)-insulin at pH 7.0. Biochim. Biophys. Acta. 1977;495:195–202. - PubMed
-
- Attri AK, Minton AP. New methods for measuring macromolecular interactions in solution via static light scattering: basic methodology and application to nonassociating and self-associating proteins. Anal. Biochem. 2005;337:103–110. - PubMed
-
- Attri AK, Minton AP. Composition gradient static light scattering: a new technique for rapid detection and quantitative characterization of reversible macromolecular hetero-associations in solution. Anal. Biochem. 2005;346:132–138. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

