Peripheral inflammation induces tumor necrosis factor dependent AMPA receptor trafficking and Akt phosphorylation in spinal cord in addition to pain behavior
- PMID: 20202754
- PMCID: PMC2860679
- DOI: 10.1016/j.pain.2010.02.008
Peripheral inflammation induces tumor necrosis factor dependent AMPA receptor trafficking and Akt phosphorylation in spinal cord in addition to pain behavior
Abstract
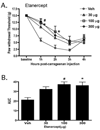
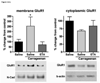
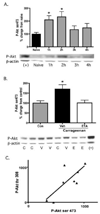
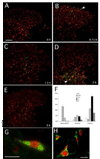
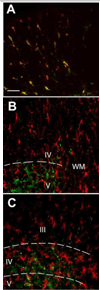
In the present study, intraplantar carrageenan induced increased mechanical allodynia, phosphorylation of PKB/Akt and GluR1 ser 845 (PKA site) as well as GluR1, but not GluR2 movement into neuronal membranes. This change in membrane GluR1/GluR2 ratio is indicative of Ca(2+) permeable AMPA receptor insertion. Pain behavior was reduced and biochemical changes blocked by spinal pretreatment, but not post-treatment, with a tumor necrosis factor (TNF) antagonist, Etanercept (100microg). Pain behavior was also reduced by spinal inhibition of phosphatidylinositol 3-kinase (PI-3K) (wortmannin; 1 and 5microg) and LY294002; 50 and 100microg) and Akt (Akt inhibitor IV; 3microg). Phosphorylated Akt was found exclusively in neurons in grey matter and in oligodendrocytes in white matter. Interestingly, this increase was seen first in superficial dorsal horn and alpha-motor neurons (peak 45min) and later (peak 2h post-injection) in deep dorsal horn neurons. Akt and GluR1 phosphorylation, AMPA receptor trafficking and mechanical allodynia were all TNF dependent. Whether phosphorylation of Akt and of GluR1 are in series or in parallel or upstream of pain behavior remains to be determined. Certainly, TNF-mediated GluR1 trafficking appears to play a major role in inflammatory pain and TNF-mediated effects such as these could represent a path by which glia contribute to neuronal sensitization (spinal LTP) and pathological pain.
Copyright 2010 International Association for the Study of Pain. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
CONFLICT OF INTEREST
None of the authors has a conflict of interest with the contents of this paper.
Figures





Similar articles
-
Inflammation-induced GluA1 trafficking and membrane insertion of Ca2+ permeable AMPA receptors in dorsal horn neurons is dependent on spinal tumor necrosis factor, PI3 kinase and protein kinase A.Exp Neurol. 2017 Jul;293:144-158. doi: 10.1016/j.expneurol.2017.04.004. Epub 2017 Apr 12. Exp Neurol. 2017. PMID: 28412220 Free PMC article.
-
Carrageenan induced phosphorylation of Akt is dependent on neurokinin-1 expressing neurons in the superficial dorsal horn.Mol Pain. 2012 Jan 13;8:4. doi: 10.1186/1744-8069-8-4. Mol Pain. 2012. PMID: 22243518 Free PMC article.
-
Surgical incision induces phosphorylation of AMPA receptor GluR1 subunits at Serine-831 sites and GluR1 trafficking in spinal cord dorsal horn via a protein kinase Cγ-dependent mechanism.Neuroscience. 2013 Jun 14;240:361-70. doi: 10.1016/j.neuroscience.2013.02.051. Epub 2013 Mar 5. Neuroscience. 2013. PMID: 23470774
-
Dorsal horn alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking in inflammatory pain.Anesthesiology. 2010 May;112(5):1259-65. doi: 10.1097/ALN.0b013e3181d3e1ed. Anesthesiology. 2010. PMID: 20395828 Free PMC article. Review.
-
Regulation of AMPA receptors in spinal nociception.Mol Pain. 2010 Jan 21;6:5. doi: 10.1186/1744-8069-6-5. Mol Pain. 2010. PMID: 20092646 Free PMC article. Review.
Cited by
-
Resolvin D1 accelerates resolution of neuroinflammation by inhibiting microglia activation through the BDNF/TrkB signaling pathway.Eur J Med Res. 2025 Mar 20;30(1):189. doi: 10.1186/s40001-025-02424-7. Eur J Med Res. 2025. PMID: 40114280 Free PMC article.
-
Mammalian target of rapamycin in spinal cord neurons mediates hypersensitivity induced by peripheral inflammation.Neuroscience. 2010 Sep 1;169(3):1392-402. doi: 10.1016/j.neuroscience.2010.05.067. Epub 2010 Jun 9. Neuroscience. 2010. PMID: 20538043 Free PMC article.
-
The Central Role of Glia in Pathological Pain and the Potential of Targeting the Cannabinoid 2 Receptor for Pain Relief.ISRN Anesthesiol. 2011;2011(2011):593894. doi: 10.5402/2011/593894. ISRN Anesthesiol. 2011. PMID: 22442754 Free PMC article.
-
Hippocampal TNFα Signaling Contributes to Seizure Generation in an Infection-Induced Mouse Model of Limbic Epilepsy.eNeuro. 2017 May 9;4(2):ENEURO.0105-17.2017. doi: 10.1523/ENEURO.0105-17.2017. eCollection 2017 Mar-Apr. eNeuro. 2017. PMID: 28497109 Free PMC article.
-
Maladaptive spinal plasticity opposes spinal learning and recovery in spinal cord injury.Front Physiol. 2012 Oct 10;3:399. doi: 10.3389/fphys.2012.00399. eCollection 2012. Front Physiol. 2012. PMID: 23087647 Free PMC article.
References
-
- Barry MF, Ziff EB. Receptor trafficking and the plasticity of excitatory synapses. Curr Opin Neurobiol. 2002;12:279–286. - PubMed
-
- Beattie EC, Carroll RC, Yu X, Morishita W, Yasuda H, von Zastrow M, Malenka RC. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat Neurosci. 2000;3:1291–1300. - PubMed
-
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–2285. - PubMed
-
- Burnashev N, Monyer H, Seeburg PH, Sakmann B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron. 1992;8:189–198. - PubMed
-
- Carlton SM, Hargett GL, Coggeshall RE. Plasticity in alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor subunits in the rat dorsal horn following deafferentation. Neurosci Lett. 1998;242:21–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

