Structure, biological functions and applications of the AB5 toxins
- PMID: 20202851
- PMCID: PMC2929601
- DOI: 10.1016/j.tibs.2010.02.003
Structure, biological functions and applications of the AB5 toxins
Abstract
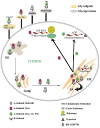
AB(5) toxins are important virulence factors for several major bacterial pathogens, including Bordetella pertussis, Vibrio cholerae, Shigella dysenteriae and at least two distinct pathotypes of Escherichia coli. The AB(5) toxins are so named because they comprise a catalytic A-subunit, which is responsible for disruption of essential host functions, and a pentameric B-subunit that binds to specific glycan receptors on the target cell surface. The molecular mechanisms by which the AB(5) toxins cause disease have been largely unravelled, including recent insights into a novel AB(5) toxin family, subtilase cytotoxin (SubAB). Furthermore, AB(5) toxins have become a valuable tool for studying fundamental cellular functions, and are now being investigated for potential applications in the clinical treatment of human diseases.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures

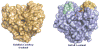
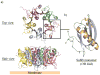
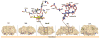
References
-
- Sack DA, et al. Cholera. Lancet. 2004;363:223–233. - PubMed
-
- Tarr PI, et al. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet. 2005;365:1073–1086. - PubMed
-
- Griffin P. Shiga toxin-producing Escherichia coli infections in the United States. 4th International Symposium and Workshop on Shiga Toxin-producing Escherichia coli Infections. 2000:21.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

