Expanding the range of 'druggable' targets with natural product-based libraries: an academic perspective
- PMID: 20202892
- PMCID: PMC2878877
- DOI: 10.1016/j.cbpa.2010.02.001
Expanding the range of 'druggable' targets with natural product-based libraries: an academic perspective
Abstract
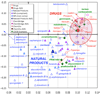
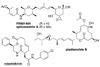
Existing drugs address a relatively narrow range of biological targets. As a result, libraries of drug-like molecules have proven ineffective against a variety of challenging targets, such as protein-protein interactions, nucleic acid complexes, and antibacterial modalities. In contrast, natural products are known to be effective at modulating such targets, and new libraries are being developed based on underrepresented scaffolds and regions of chemical space associated with natural products. This has led to several recent successes in identifying new chemical probes that address these challenging targets.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures


References
-
-
Lazo JS, Brady LS, Dingledine R. Building a pharmacological lexicon: small molecule discovery in academia. Mol Pharmacol. 2007;72:1–7. • An excellent overview of academic screening in the context of the NIH Molecular Libraries Screening Center Network (MLSCN).
-
-
-
Overington JP, Al-Lazikani B, Hopkins AL. How many drug targets are there? Nat Rev Drug Discov. 2006;5:993–996. •• A comprehensive effort to identify and classify the biological targets of all existing drugs. One key finding is that small molecule drugs modulate only 207 targets encoded in the human genome.
-
-
- Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discov. 2002;1:727–730. - PubMed
-
-
Dobson CM. Chemical space and biology. Nature. 2004;432:824–828. • An excellent introduction to the concept of chemical space and its interactions with biology.
-
-
- Bohacek RS, McMartin C, Guida WC. The art and practice of structure-based drug design: A molecular modeling perspective. Med Res Rev. 1996;16:3–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

