An intersubunit interaction between S4-S5 linker and S6 is responsible for the slow off-gating component in Shaker K+ channels
- PMID: 20202932
- PMCID: PMC2859562
- DOI: 10.1074/jbc.M109.097717
An intersubunit interaction between S4-S5 linker and S6 is responsible for the slow off-gating component in Shaker K+ channels
Abstract
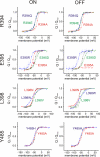
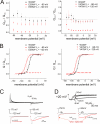
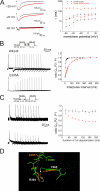
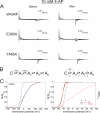
Voltage-gated ion channels are controlled by the membrane potential, which is sensed by peripheral, positively charged voltage sensors. The movement of the charged residues in the voltage sensor may be detected as gating currents. In Shaker K(+) channels, the gating currents are asymmetric; although the on-gating currents are fast, the off-gating currents contain a slow component. This slow component is caused by a stabilization of the activated state of the voltage sensor and has been suggested to be linked to ion permeation or C-type inactivation. The molecular determinants responsible for the stabilization, however, remain unknown. Here, we identified an interaction between Arg-394, Glu-395, and Leu-398 on the C termini of the S4-S5 linker and Tyr-485 on the S6 of the neighboring subunit, which is responsible for the development of the slow off-gating component. Mutation of residues involved in this intersubunit interaction modulated the strength of the associated interaction. Impairment of the interaction still led to pore opening but did not exhibit slow gating kinetics. Development of this interaction occurs under physiological ion conduction and is correlated with pore opening. We, thus, suggest that the above residues stabilize the channel in the open state.
Figures




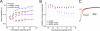
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

