Novel mode of phosphorylation-triggered reorganization of the nuclear lamina during nuclear egress of human cytomegalovirus
- PMID: 20202933
- PMCID: PMC2859560
- DOI: 10.1074/jbc.M109.063628
Novel mode of phosphorylation-triggered reorganization of the nuclear lamina during nuclear egress of human cytomegalovirus
Abstract
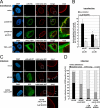
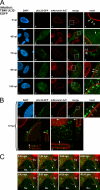
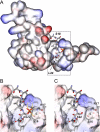
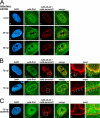
The nucleocytoplasmic egress of viral capsids is a rate-limiting step in the replication of the human cytomegalovirus (HCMV). As reported recently, an HCMV-specific nuclear egress complex is composed of viral and cellular proteins, in particular protein kinases with the capacity to induce destabilization of the nuclear lamina. Viral protein kinase pUL97 and cellular protein kinase C (PKC) play important roles by phosphorylating several types of nuclear lamins. Using pUL97 mutants, we show that the lamin-phosphorylating activity of pUL97 is associated with a reorganization of nuclear lamin A/C. Either pUL97 or PKC has the potential to induce distinct punctate lamina-depleted areas at the periphery of the nuclear envelope, which were detectable in transiently transfected and HCMV-infected cells. Using recombinant HCMV, which produces green fluorescent protein-labeled viral capsids, the direct transition of viral capsids through these areas could be visualized. This process was sensitive to an inhibitor of pUL97/PKC activity. The pUL97-mediated phosphorylation of lamin A/C at Ser(22) generated a novel binding motif for the peptidyl-prolyl cis/trans-isomerase Pin1. In HCMV-infected fibroblasts, the physiological localization of Pin1 was altered, leading to recruitment of Pin1 to viral replication centers and to the nuclear lamina. The local increase in Pin1 peptidyl-prolyl cis/trans-isomerase activity may promote conformational modulation of lamins. Thus, we postulate a novel phosphorylation-triggered mechanism for the reorganization of the nuclear lamina in HCMV-infected cells.
Figures



Similar articles
-
The Prolyl Isomerase Pin1 Promotes the Herpesvirus-Induced Phosphorylation-Dependent Disassembly of the Nuclear Lamina Required for Nucleocytoplasmic Egress.PLoS Pathog. 2016 Aug 24;12(8):e1005825. doi: 10.1371/journal.ppat.1005825. eCollection 2016 Aug. PLoS Pathog. 2016. PMID: 27556400 Free PMC article.
-
Regulatory roles of protein kinases in cytomegalovirus replication.Adv Virus Res. 2011;80:69-101. doi: 10.1016/B978-0-12-385987-7.00004-X. Adv Virus Res. 2011. PMID: 21762822 Review.
-
Human Cytomegalovirus Nuclear Capsids Associate with the Core Nuclear Egress Complex and the Viral Protein Kinase pUL97.Viruses. 2018 Jan 13;10(1):35. doi: 10.3390/v10010035. Viruses. 2018. PMID: 29342872 Free PMC article.
-
The peptidyl-prolyl cis/trans isomerase Pin1 interacts with three early regulatory proteins of human cytomegalovirus.Virus Res. 2020 Aug;285:198023. doi: 10.1016/j.virusres.2020.198023. Epub 2020 May 16. Virus Res. 2020. PMID: 32428517
-
Clinical development of letermovir and maribavir: Overview of human cytomegalovirus drug resistance.Antiviral Res. 2019 Mar;163:91-105. doi: 10.1016/j.antiviral.2019.01.011. Epub 2019 Jan 25. Antiviral Res. 2019. PMID: 30690043 Review.
Cited by
-
RASCAL is a new human cytomegalovirus-encoded protein that localizes to the nuclear lamina and in cytoplasmic vesicles at late times postinfection.J Virol. 2010 Jul;84(13):6483-96. doi: 10.1128/JVI.02462-09. Epub 2010 Apr 14. J Virol. 2010. PMID: 20392852 Free PMC article.
-
High-resolution crystal structures of two prototypical β- and γ-herpesviral nuclear egress complexes unravel the determinants of subfamily specificity.J Biol Chem. 2020 Mar 6;295(10):3189-3201. doi: 10.1074/jbc.RA119.011546. Epub 2020 Jan 24. J Biol Chem. 2020. PMID: 31980459 Free PMC article.
-
Assessment of Covalently Binding Warhead Compounds in the Validation of the Cytomegalovirus Nuclear Egress Complex as an Antiviral Target.Cells. 2023 Apr 14;12(8):1162. doi: 10.3390/cells12081162. Cells. 2023. PMID: 37190072 Free PMC article.
-
Transmembrane Protein pUL50 of Human Cytomegalovirus Inhibits ISGylation by Downregulating UBE1L.J Virol. 2018 Jul 17;92(15):e00462-18. doi: 10.1128/JVI.00462-18. Print 2018 Aug 1. J Virol. 2018. PMID: 29743376 Free PMC article.
-
Lamin post-translational modifications: emerging toggles of nuclear organization and function.Trends Biochem Sci. 2021 Oct;46(10):832-847. doi: 10.1016/j.tibs.2021.05.007. Epub 2021 Jun 18. Trends Biochem Sci. 2021. PMID: 34148760 Free PMC article. Review.
References
-
- Mocarski E. S., Shenk T., Pass R. F. (2007) in Fields Virology (Knipe D. M., Howley P. M. eds) pp. 2701–2772, Lippincott/Williams & Wilkins, Philadelphia, PA
-
- Vancíková Z., Dvorák P. (2001) Curr. Drug Targets Immune Endocr. Metab. Disord. 1, 179–187 - PubMed
-
- Mettenleiter T. C., Klupp B. G., Granzow H. (2009) Virus Res. 143, 222–234 - PubMed
-
- Sanchez V., Spector D. H. (2002) Science 297, 778–779 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

