On the relevance of the Met-turn methionine in metzincins
- PMID: 20202937
- PMCID: PMC2859557
- DOI: 10.1074/jbc.M109.083378
On the relevance of the Met-turn methionine in metzincins
Abstract
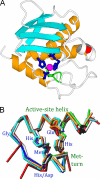
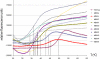
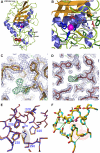
The metzincins are a clan of metallopeptidases consisting of families that share a series of structural elements. Among them is the Met-turn, a tight 1,4-turn found directly below the zinc-binding site, which is structurally and spatially conserved and invariantly shows a methionine at position 3 in all metzincins identified. The reason for this conservation has been a matter of debate since its discovery. We have studied this structural element in Methanosarcina acetivorans ulilysin, the structural prototype of the pappalysin family, by generating 10 mutants that replaced methionine with proteogenic amino acids. We compared recombinant overexpression yields, autolytic and tryptic activation, proteolytic activity, thermal stability, and three-dimensional structure with those of the wild type. All forms were soluble and could be purified, although with varying yields, and three variants underwent autolysis, could be activated by trypsin, and displayed significant proteolytic activity. All variants were analyzed for the thermal stability of their zymogens. None of the mutants analyzed proved more stable or active than the wild type. Both bulky and small side chains, as well as hydrophilic ones, showed diminished thermal stability. Two mutants, leucine and cysteine, crystallized and showed three-dimensional structures that were indistinguishable from the wild type. These studies reveal that the Met-turn acts as a plug that snugly inserts laterally into a core structure created by the protein segment engaged in zinc binding and thus contributes to its structural integrity, which is indispensable for function. Replacement of the methionine with residues that deviate in size, side-chain conformation, and chemical properties impairs the plug-core interaction and prejudices molecular stability and activity.
Figures
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Miscellaneous

