Functional oligomerization of the Saccharomyces cerevisiae isoprenylcysteine carboxyl methyltransferase, Ste14p
- PMID: 20202940
- PMCID: PMC2859497
- DOI: 10.1074/jbc.M109.061366
Functional oligomerization of the Saccharomyces cerevisiae isoprenylcysteine carboxyl methyltransferase, Ste14p
Abstract
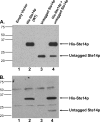
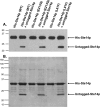
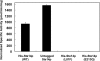
The isoprenylcysteine carboxyl methyltransferase (Icmt) from Saccharomyces cerevisiae, also designated Ste14p, is a 26-kDa integral membrane protein that contains six transmembrane spanning segments. This protein is localized to the endoplasmic reticulum membrane where it performs the methylation step of the CAAX post-translational processing pathway. Sequence analysis reveals a putative GXXXG dimerization motif located in transmembrane 1 of Ste14p, but it is not known whether Ste14p forms or functions as a dimer or higher order oligomer. We determined that Ste14p predominantly formed a homodimer in the presence of the cross-linking agent, bis-sulfosuccinimidyl suberate. Wild-type untagged Ste14p also co-immunoprecipitated and co-purified with N-terminal-tagged His(10)-myc(3)-Ste14p (His-Ste14p). Furthermore, enzymatically inactive His-Ste14p variants L81F and E213Q both exerted a dominant-negative effect on methyltransferase activity when co-expressed and co-purified with untagged wild-type Ste14p. Together, these data, although indirect, suggest that Ste14p forms and functions as a homodimer or perhaps a higher oligomeric species.
Figures

References
-
- Clarke S. (1992) Annu. Rev. Biochem. 61, 355–386 - PubMed
-
- Zhang F. L., Casey P. J. (1996) Annu. Rev. Biochem. 65, 241–269 - PubMed
-
- Young S. G., Ambroziak P., Kim E., Clarke S. (2000) The Enzymes: Protein Lipidation (Tamanoi F., Sigman D. S. eds) Vol. 21, pp. 155–213, Academic Press, San Diego, CA
-
- Hrycyna C. A., Clarke S. (1993) Pharmacol. Ther. 59, 281–300 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

