Daily dosing of tacrolimus in patients treated with HIV-1 therapy containing a ritonavir-boosted protease inhibitor or raltegravir
- PMID: 20202988
- PMCID: PMC2902821
- DOI: 10.1093/jac/dkq054
Daily dosing of tacrolimus in patients treated with HIV-1 therapy containing a ritonavir-boosted protease inhibitor or raltegravir
Abstract
Objectives: The number of HIV-infected patients receiving orthotopic liver transplantation (OLTX) is increasing. One major challenge is the severe drug-drug interactions between immunosuppressive drugs such as tacrolimus and ritonavir-boosted HIV-1 protease inhibitors (PIs). The introduction of raltegravir, which is not metabolized by the cytochrome system, may allow concomitant treatment without dose adaptation.
Patients and methods: We conducted a retrospective analysis of HIV-1-infected patients receiving tacrolimus concomitantly with different HIV therapies, including 12 h pharmacokinetic assessment of drug levels.
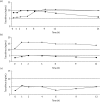
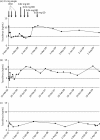
Results: Three OLTX patients received a ritonavir-boosted PI therapy when tacrolimus was added at very low doses of 0.06, 0.03 and 0.08 mg daily. Median tacrolimus blood levels were 6.6, 3.0 and 7.9 ng/mL over a follow-up period of 8, 22 and 33 months, respectively. In two other patients (one after OLTX and one with Crohn's disease), a raltegravir-based HIV therapy was started while patients received 1 or 2 mg of tacrolimus twice daily. No tacrolimus dose adjustment was necessary and drug levels remained unchanged.
Conclusions: Decreasing the dose of tacrolimus to 0.03-0.08 mg daily in patients with concomitant boosted PI therapy resulted in stable tacrolimus blood levels without alteration of PI drug levels. Concomitant use of raltegravir and tacrolimus revealed no clinically relevant drug interaction.
Figures
Similar articles
-
Efficacy and safety of once daily elvitegravir versus twice daily raltegravir in treatment-experienced patients with HIV-1 receiving a ritonavir-boosted protease inhibitor: randomised, double-blind, phase 3, non-inferiority study.Lancet Infect Dis. 2012 Jan;12(1):27-35. doi: 10.1016/S1473-3099(11)70249-3. Epub 2011 Oct 18. Lancet Infect Dis. 2012. PMID: 22015077 Clinical Trial.
-
Substitution of raltegravir for ritonavir-boosted protease inhibitors in HIV-infected patients: the SPIRAL study.AIDS. 2010 Jul 17;24(11):1697-707. doi: 10.1097/QAD.0b013e32833a608a. AIDS. 2010. PMID: 20467288 Clinical Trial.
-
Plasma and intracellular pharmacokinetics of darunavir/ritonavir once daily and raltegravir once and twice daily in HIV-infected individuals.J Acquir Immune Defic Syndr. 2011 Dec 15;58(5):450-7. doi: 10.1097/QAI.0b013e3182364c67. J Acquir Immune Defic Syndr. 2011. PMID: 21926632 Free PMC article. Clinical Trial.
-
Lopinavir/ritonavir: a review of its use in the management of HIV infection.Drugs. 2003;63(8):769-802. doi: 10.2165/00003495-200363080-00004. Drugs. 2003. PMID: 12662125 Review.
-
Novel antiretroviral combinations in treatment-experienced patients with HIV infection: rationale and results.Drugs. 2010 Sep 10;70(13):1629-42. doi: 10.2165/11538020-000000000-00000. Drugs. 2010. PMID: 20731472 Review.
Cited by
-
Best single time point correlations with AUC for cyclosporine and tacrolimus in HIV-infected kidney and liver transplant recipients.Transplantation. 2014 Mar 27;97(6):702-7. doi: 10.1097/01.TP.0000441097.30094.31. Transplantation. 2014. PMID: 24389906 Free PMC article.
-
Drug-related Problems in Solid-Organ Transplant Recipients Hospitalized for COVID-19: An Experience of a Referral Tertiary Center in Iran.Iran J Med Sci. 2022 Nov;47(6):577-587. doi: 10.30476/IJMS.2022.93366.2467. Iran J Med Sci. 2022. PMID: 36380982 Free PMC article.
-
Organ Transplantation in HIV Patients: Current Status and New Directions.Curr Infect Dis Rep. 2013 Dec;15(6):526-35. doi: 10.1007/s11908-013-0381-x. Curr Infect Dis Rep. 2013. PMID: 24142801
-
Nirmatrelvir/ritonavir treatment in SARS-CoV-2 positive kidney transplant recipients - a case series with four patients.BMC Nephrol. 2023 Apr 15;24(1):99. doi: 10.1186/s12882-023-03154-w. BMC Nephrol. 2023. PMID: 37061677 Free PMC article.
-
Coronavirus Disease 2019 (COVID-19) and Transplantation: Pharmacotherapeutic Management of Immunosuppression Regimen.Ther Clin Risk Manag. 2020 Jul 3;16:617-629. doi: 10.2147/TCRM.S256246. eCollection 2020. Ther Clin Risk Manag. 2020. PMID: 32694915 Free PMC article. Review.
References
-
- Sattler M, Guengerich FP, Yun CH, et al. Cytochrome P-450 3A enzymes are responsible for biotransformation of FK506 and rapamycin in man and rat. Drug Metab Dispos. 1992;20:753–61. - PubMed
-
- Vincent SH, Karanam BV, Painter SK, et al. In vitro metabolism of FK-506 in rat, rabbit, and human liver microsomes: identification of a major metabolite and of cytochrome P450 3A as the major enzymes responsible for its metabolism. Arch Biochem Biophys. 1992;294:454–60. - PubMed
-
- Paterson DL, Singh N. Interactions between tacrolimus and antimicrobial agents. Clin Infect Dis. 1997;25:1430–40. - PubMed
-
- Christians U, Jacobsen W, Benet LZ, et al. Mechanisms of clinically relevant drug interactions associated with tacrolimus. Drug Metab Dispos. 2003;31:1292–5. - PubMed
-
- van Heeswijk RP, Veldkamp A, Mulder JW, et al. Combination of protease inhibitors for the treatment of HIV-1-infected patients: a review of pharmacokinetics and clinical experience. Antivir Ther. 2001;6:201–29. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

