Crucial role of the small GTPase Rac1 in insulin-stimulated translocation of glucose transporter 4 to the mouse skeletal muscle sarcolemma
- PMID: 20203090
- PMCID: PMC4183928
- DOI: 10.1096/fj.09-137380
Crucial role of the small GTPase Rac1 in insulin-stimulated translocation of glucose transporter 4 to the mouse skeletal muscle sarcolemma
Abstract
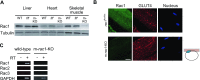
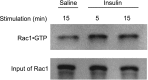
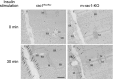
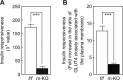
The Rho family GTPase Rac1 has been implicated in the regulation of glucose uptake in myoblast cell lines. However, no evidence for the role of Rac1 has been provided by a mouse model. The purpose of this study is to test the involvement of Rac1 in insulin action in mouse skeletal muscle. Intravenous administration of insulin indeed elicited Rac1 activation in gastrocnemius muscle, suggesting the involvement of Rac1 in this signaling pathway. We then examined whether insulin-stimulated translocation of the facilitative glucose transporter GLUT4 from its storage sites to the skeletal muscle sarcolemma depends on Rac1. We show that ectopic expression of constitutively activated Rac1, as well as intravenous administration of insulin, caused translocation of GLUT4 to the gastrocnemius muscle sarcolemma, as revealed by immunofluorescent staining of a transiently expressed exofacial epitope-tagged GLUT4 reporter. Of particular note, insulin-dependent, but not constitutively activated Rac1-induced, GLUT4 translocation was markedly suppressed in skeletal muscle-specific rac1-knockout mice compared to control mice. Immunogold electron microscopic analysis of endogenous GLUT4 gave similar results. Collectively, we propose a critical role of Rac1 in insulin-dependent GLUT4 translocation to the skeletal muscle sarcolemma, which has heretofore been predicted solely by cell culture studies.
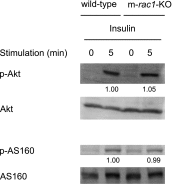
Figures

References
-
- Klip A., Pâquet M. R. (1990) Glucose transport and glucose transporters in muscle and their metabolic regulation. Diabetes Care 13, 228–243 - PubMed
-
- Saltiel A. R., Kahn C. R. (2001) Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414, 799–806 - PubMed
-
- Biddinger S. B., Kahn C. R. (2006) From mice to men: insights into the insulin resistance syndromes. Annu. Rev. Physiol. 68, 123–158 - PubMed
-
- Taniguchi C. M., Emanuelli B., Kahn C. R. (2006) Critical nodes in signalling pathways: insights into insulin action. Nat. Rev. Mol. Cell. Biol. 7, 85–96 - PubMed
-
- Huang S., Czech M. P. (2007) The GLUT4 glucose transporter. Cell Metab. 5, 237–252 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

